Global Exosome Diagnostic and Therapeutic Market By Product (Instrument, Reagent, Software) By Application (Diagnostic, Therapeutic) By End User (Cancer Institute, Hospital, Diagnostic Center, Others) Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: June 2024
- Report ID: 151255
- Number of Pages: 349
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
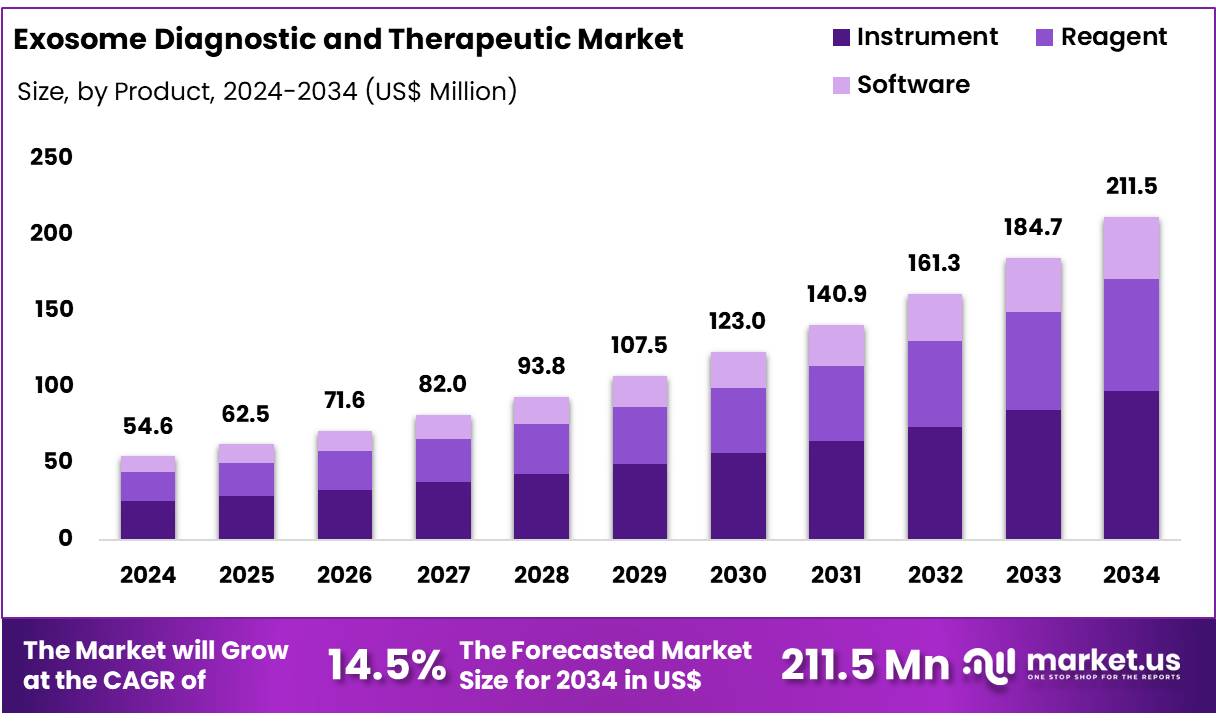
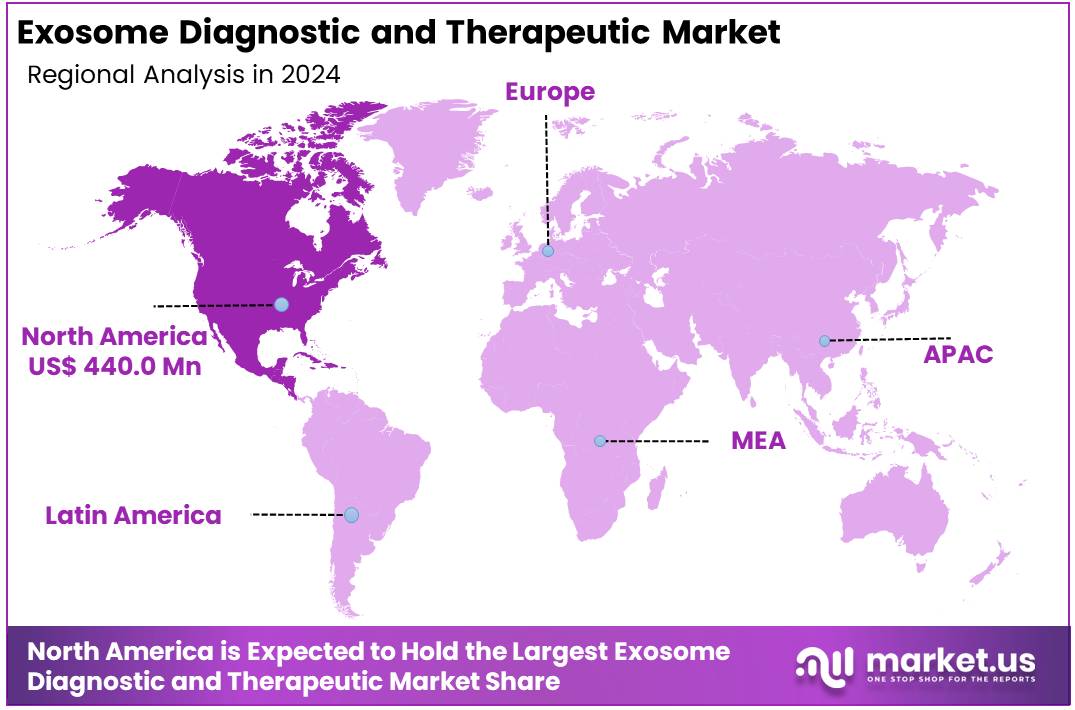
Global Exosome Diagnostic and Therapeutic Market size is expected to be worth around US$ 211.5 Million by 2034 from US$ 54.6 Million in 2024, growing at a CAGR of 14.5% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 45.5% share with a revenue of US$ 440.0 Million.
A groundbreaking platform leveraging exosomes naturally occurring extracellular vesicles approximately 30–150 nm in size is being advanced to enhance disease detection and treatment delivery. These vesicles, secreted by most eukaryotic cells, carry proteins, lipids, and nucleic acids that reflect the state of their cell of origin and can be isolated from blood, urine, and other biofluids for clinical use.
The diagnostic arm of the platform enables early-stage disease detection by profiling exosomal protein and RNA biomarkers associated with cancer, neurodegenerative disorders, and cardiovascular conditions. Assay performance is being validated under strict FDA guidelines for diagnostic assays, ensuring sensitivity and specificity suitable for liquid biopsy applications.
On the therapeutic front, exosomes are engineered to deliver mRNA, small molecules, and antibodies with high biocompatibility and targeted tropism. Preclinical studies have demonstrated efficient blood–brain barrier crossing and tissue regeneration capabilities. Regulatory pathways are being navigated in accordance with the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act to secure Investigational New Drug (IND) approvals for first-in-class exosome therapeutics.
Key Takeaways
- Market Size: Global Exosome Diagnostic and Therapeutic Market size is expected to be worth around US$ 211.5 Million by 2034 from US$ 54.6 Million in 2024.
- Market Growth: The market growing at a CAGR of 14.5% during the forecast period from 2025 to 2034.Product Analysis: In 2024, the instrument segment dominated the exosome diagnostic and therapeutic market with a 45.4% share.
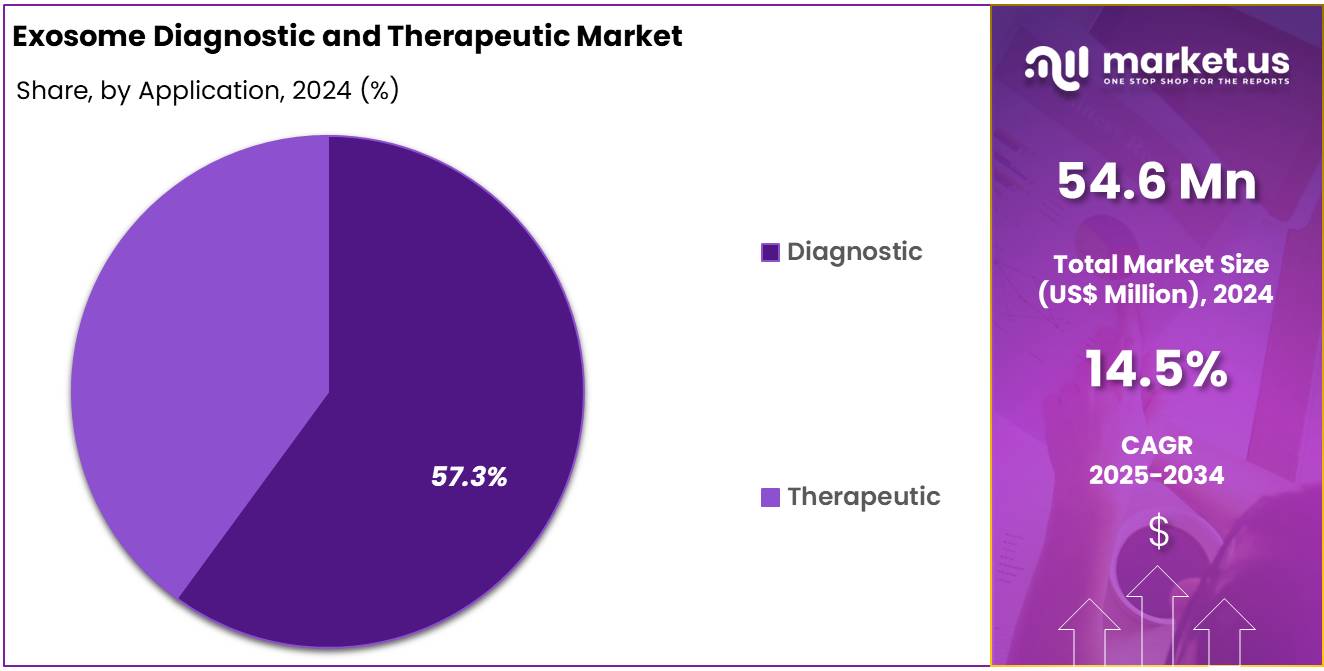
- Application Analysis: The diagnostic application segment was observed to dominate the exosome market, accounting for 57.3% of total use.
- End-Use Analysis: In 2024, the cancer institute end-user segment was observed to dominate the exosome diagnostic and therapeutic market, accounting for 37.4% of total uptake.
- Regional Analysis: In 2024, North America led the market, achieving over 45.5% share with a revenue of US$ 440.0 Million.
Product Analysis
In 2024, the instrument segment dominated the exosome diagnostic and therapeutic market with a 45.4% share. This category encompasses ultracentrifuges, nanoparticle tracking analyzers, flow cytometers and microfluidic isolation platforms. These devices are subject to FDA quality systems regulations under 21 CFR Part 820 to ensure consistent exosome isolation and characterization performance.
The reagent segment accounting for approximately 35% of the market includes exosome enrichment kits, affinity-capture reagents, lysis buffers and RNA extraction kits. Standardization of reagents in NIH-funded studies has enhanced assay reproducibility and biomarker quantification accuracy.
Software solutions comprise the remaining market share. These platforms enable high-throughput data analysis, bioinformatic processing of exosomal RNA sequencing data and machine-learning–driven biomarker discovery. FDA guidance on software as a medical device (SaMD) underpins clinical validation, cybersecurity and interoperability with laboratory information management systems.
Together, the robust performance of instruments, the consistency of reagents and the analytical power of software form an integrated product ecosystem that supports comprehensive exosome workflows from sample preparation through data interpretation driving sustained market growth in both research and clinical applications.
Application Analysis
The diagnostic application segment was observed to dominate the exosome market, accounting for 57.3% of total use. Clinical applications were primarily focused on non-invasive disease detection. Over 288 exosome-based diagnostic trials had been registered on ClinicalTrials.gov by the end of 2023, spanning oncology, cardiovascular, and neurodegenerative indications.
Cancer diagnostics comprised more than half of these studies, leveraging exosomal miRNA and protein biomarkers to enhance early detection and prognostic accuracy. Liquid biopsy platforms incorporating exosome cargo have been developed under FDA in vitro diagnostic guidance, with pilot studies reporting sensitivities above 90%.
Therapeutic applications represented the remaining 42.7% of market activity. These efforts are centered on engineered exosomes for targeted drug delivery and regenerative medicine. To date, 59 exosome-based therapeutic trials have been listed on ClinicalTrials.gov, addressing conditions such as myocardial infarction, autoimmune disorders, and acute respiratory distress syndrome. Preclinical models have demonstrated that mesenchymal stem cell-derived exosomes promote tissue repair and modulate immune responses, supporting progression toward clinical translation.
End User Analysis
In 2024, the cancer institute end-user segment was observed to dominate the exosome diagnostic and therapeutic market, accounting for 37.4% of total uptake. Adoption was concentrated among the 73 NCI-designated Cancer Centers, which are funded and overseen by the National Cancer Institute to conduct cutting-edge clinical and translational research.
Hospitals represented the second-largest end-user group, with a 28.9% share. Over 6,093 U.S. hospitals—including community, teaching and government facilities maintained clinical laboratories that integrated exosome assays into routine diagnostic workflows under CLIA regulations.
Diagnostic centers held 22.1% of the market. More than 12,000 Medicare-approved clinical laboratories performed exosome isolation and biomarker profiling services in 2024, as reported by CMS provider enrollment data.
The remaining 11.6% comprised “Others,” including academic research institutes, biobanking facilities and contract research organizations. These entities leveraged exosome platforms for preclinical studies, biomarker discovery and early-phase therapeutic development, thereby contributing to the overall ecosystem of exosome-based innovation.
Key Market Segments
By Product
- Instrument
- Reagent
- Software
By Application
- Diagnostic
- Therapeutic
By End User
- Cancer Institute
- Hospital
- Diagnostic Center
- Others
Driving Factors
The growth of the Exosome Diagnostic and Therapeutic market can be attributed to substantial government research funding. Since 2013, the NIH Common Fund’s Extracellular RNA Communication program has been supported with over $145 million to accelerate exRNA biology and technology development for diagnostics and therapeutics.
This investment has enabled the creation of advanced isolation methods, analytical tools, and biofluid reference profiles. In addition, foundational knowledge concerning exRNA carriers has been produced, thereby facilitating translational applications such as biomarker assays and therapeutic delivery platforms. Consequently, increased grant support has catalyzed both basic research and commercial interest, driving market expansion.
Trending Factors
A significant trend in the market is the emergence of exosome-based liquid biopsy methods for non-invasive disease detection. Recently, exosomes have been demonstrated to offer real-time molecular profiling by transporting proteins, lipids, and nucleic acids reflective of their cells of origin. These vesicles exhibit stability in bodily fluids, allowing for consistent monitoring of disease progression and treatment response in oncology and neurodegenerative disorders.
Microfluidic devices, nanoplasmonic chips, and magneto-electrochemical sensors have been developed to improve sensitivity and throughput. As a result, liquid biopsy is increasingly embraced by clinical laboratories, enhancing early diagnosis and personalized medicine applications within the exosome diagnostics sector.
Restraining Factors
The market’s growth is constrained by regulatory uncertainty and the absence of FDA-approved exosome products. Currently, no exosome-based diagnostics or therapeutics have received marketing authorization from the FDA. Clinics offering unapproved exosome treatments continue to be subject to warning letters and public safety alerts due to adverse events and misleading claims.
Rigorous pre-market review and compliance with drug and biologics regulations are required to establish safety and efficacy. Until standardized manufacturing practices and clinical validation studies are completed, delays in regulatory approval will continue to restrain product commercialization and broader clinical adoption.
Opportunity
Considerable opportunity exists in exosome-mediated targeted drug delivery and regenerative medicine. Exosomes’ intrinsic biocompatibility, low immunogenicity, and ability to cross biological barriers render them ideal carriers for chemotherapeutics, nucleic acids, and proteins. Preclinical studies have demonstrated effective encapsulation and delivery of agents such as doxorubicin and paclitaxel to tumors, minimizing off-target effects and enhancing therapeutic index.
Furthermore, MSC-derived exosomes have been shown to promote tissue repair in cardiac injury models via anti-inflammatory and pro-angiogenic mechanisms. As manufacturing scalability and loading efficiency improve, exosome therapeutics are poised to create new treatment paradigms in oncology, cardiology, and neurology.
Regional Analysis
In 2024, North America held a dominant market position, capturing more than a 45.5% share and holds US$ 440.0 Million market value for the year. This leadership is supported by sustained federal research funding, notably the NIH’s Extracellular RNA Communication program, which has allocated over US$ 145 million since 2013 to advance exosome biology and technology. Regulatory clarity from the FDA’s Center for Biologics Evaluation and Research has enabled a growing pipeline of exosome-based clinical studies. As of mid-2024, the US registry ClinicalTrials.gov listed hundreds of exosome-related trials, most sponsored by North American institutions.
Europe’s market growth is driven by robust research grants and regulatory support. The EU’s Horizon Europe programme commits €93.5 billion to research and innovation over 2021–2027, part of which is channeled into extracellular vesicle projects. Guidance from the European Medicines Agency’s Committee for Advanced Therapies has streamlined clinical translation pathways for exosome therapeutics. National funding bodies in Germany, France, and the United Kingdom offer competitive grants for liquid biopsy and regenerative medicine applications. Comprehensive healthcare infrastructure and evolving reimbursement frameworks further bolster adoption across major EU markets.
The Asia-Pacific region is emerging as a high-growth market. China’s National Medical Products Administration (NMPA) has integrated exosome products into its biologics regulatory framework, issuing interim provisions and standards to guide clinical development and manufacturing. Government R&D agencies in Japan, South Korea, and India have launched targeted funding calls for exosome diagnostics and therapeutics. Expansion of clinical trial capacity, improvements in healthcare delivery, and increasing awareness of personalized medicine are expected to drive double-digit CAGR in APAC over the next decade.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
In the exosome diagnostic and therapeutic market, leading stakeholders can be categorized into four groups: academic research centers, contract development and manufacturing organizations (CDMOs), specialized diagnostic platform providers, and biopharmaceutical innovators. Academic research centers contribute fundamental research and early-stage biomarker discovery pipelines, supported by federal grants such as the NIH Common Fund’s Extracellular RNA Communication program.
CDMOs offer scalable vesicle isolation, purification, and formulation services under regulatory guidance from agencies like the FDA and EMA. Diagnostic platform providers develop microfluidic, nanotechnology, and immunoassay-based systems for high-sensitivity exosome detection in hospitals and clinical laboratories.
Biopharmaceutical innovators focus on exosome-mediated drug delivery and regenerative therapies, leveraging proprietary loading technologies and manufacturing processes. Competitive differentiation arises from investments in automation, assay sensitivity, and quality-control standards, as well as strategic collaborations across sectors. Regional presence and regulatory expertise also influence market share, driving partnerships to accelerate commercialization globally.
Market Key Players
- Thermo Fisher Scientific
- NanoSomix
- NX Pharmagen
- Malvern Instruments
- Capricor Therapeutic
- Exosome Diagnostics
- Exiqon A/S
- System Biosciences
- Aegle Therapeutic
- AMS Biotechnology
- Miltenyi Biotec
- Codiak BioSciences Inc.
- Lonza Group
Recent Developments
- March 2025 – Thermo Fisher Scientific entered into a definitive agreement to acquire Solventum’s Purification & Filtration business for approximately $4.1 billion in cash. This move broadens Thermo Fisher’s bioproduction capabilities, especially upstream and downstream workflows critical for exosome-based therapeutic manufacturing.
- January 2024 – Capricor Therapeutics was selected by the National Institute of Allergy and Infectious Diseases (NIAID) to conduct a Phase 1 clinical trial of its StealthX™ exosome-based multivalent COVID-19 vaccine as part of Project NextGen, reflecting government-supported validation of its platform.
- June 2023 – Malvern Instruments launched the NanoSight Pro nanoparticle tracking analysis system, featuring machine-learning-driven NS Xplorer software for ultra high-resolution exosome size/concentration measurements in under an hour.
- December 2023 – NanoSomiX was granted U.S. Patent No. 11,852,635 B2, covering its proprietary technology for quantifying exosome subpopulations and diagnosing neurodegenerative disorders. This patent underpins an upcoming commercial launch of its advanced exosome isolation and biomarker detection platform.
Report Scope
Report Features Description Market Value (2024) US$ 54.6 Million Forecast Revenue (2034) US$ 211.5 Million CAGR (2025-2034) 14.5% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Product (Instrument, Reagent, Software) By Application (Diagnostic, Therapeutic) By End User (Cancer Institute, Hospital, Diagnostic Center, Others) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Thermo Fisher Scientific, NanoSomix, NX Pharmagen, Malvern Instruments, Capricor Therapeutic, Exosome Diagnostics, Exiqon A/S, System Biosciences, Aegle Therapeutic, AMS Biotechnology, Miltenyi Biotec, Codiak BioSciences Inc., Lonza Group Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Exosome Diagnostic and Therapeutic MarketPublished date: June 2024add_shopping_cartBuy Now get_appDownload Sample
Exosome Diagnostic and Therapeutic MarketPublished date: June 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Thermo Fisher Scientific
- NanoSomix
- NX Pharmagen
- Malvern Instruments
- Capricor Therapeutic
- Exosome Diagnostics
- Exiqon A/S
- System Biosciences
- Aegle Therapeutic
- AMS Biotechnology
- Miltenyi Biotec
- Codiak BioSciences Inc.
- Lonza Group










