Global Immunology-oncology ELISA Kits Market By Product Type (Direct ELISA Kit, Indirect ELISA Kit, Sandwich ELISA Kit, and Competition or Inhibition), By Application (Cancer Diagnosis, Pharmaceutical Industry, Research and Other uses), By End-User (Hospitals, Diagnostic Centers, Research Laboratories, and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Dec 2025
- Report ID: 168585
- Number of Pages: 235
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
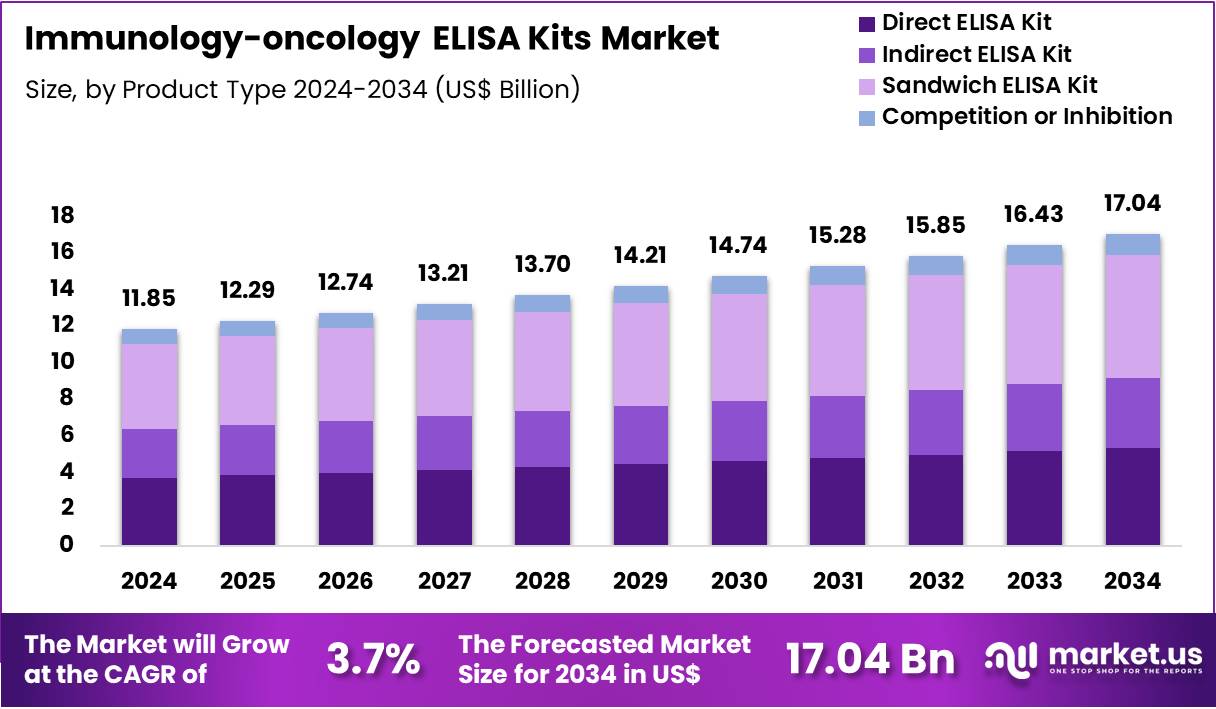
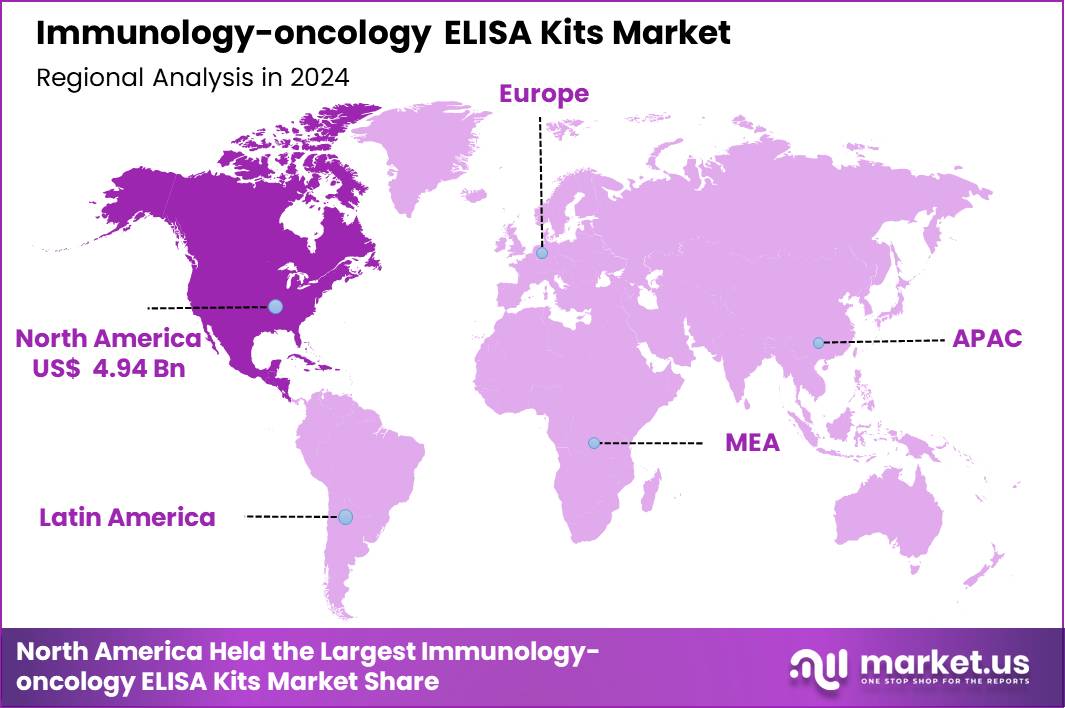
Global Immunology-oncology ELISA Kits Market size is expected to be worth around US$ 17.04 Billion by 2034 from US$ 11.85 Billion in 2024, growing at a CAGR of 3.7% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 41.7% share with a revenue of US$ 4.94 Billion.
The Immunology-Oncology ELISA Kits Market has emerged as a critical component of cancer diagnostics, immunological research, and therapeutic development. These kits enable highly specific and quantitative detection of immuno-oncology biomarkers such as cytokines, checkpoint proteins, growth factors, and tumor-associated antigens supporting clinical decision-making and translational research.
ELISA-based detection remains one of the most widely adopted immunoassay formats due to its sensitivity, reproducibility, and compatibility with high-throughput workflows. In oncology, ELISA kits assist in evaluating immune response, monitoring treatment progression, quantifying circulating immune markers, and validating therapeutic targets for immunotherapies.
The rising global burden of cancer, increased investment in immuno-oncology drugs, and demand for biomarker-driven research continue to accelerate market adoption. Diagnostic laboratories, hospitals, and pharmaceutical companies rely on these assays for validating immune pathways, supporting clinical trials, and developing companion diagnostics.
Key Takeaways
- In 2024, the market generated a revenue of US$ 85 Billion, with a CAGR of 3.7%, and is expected to reach US$ 17.04 Billion by the year 2034.
- The Kit Type segment is divided into Direct ELISA Kit, Indirect ELISA Kit, Sandwich ELISA Kit, and Competition or Inhibition, with Sandwich ELISA Kit taking the lead in 2024 with a market share of 39.6%
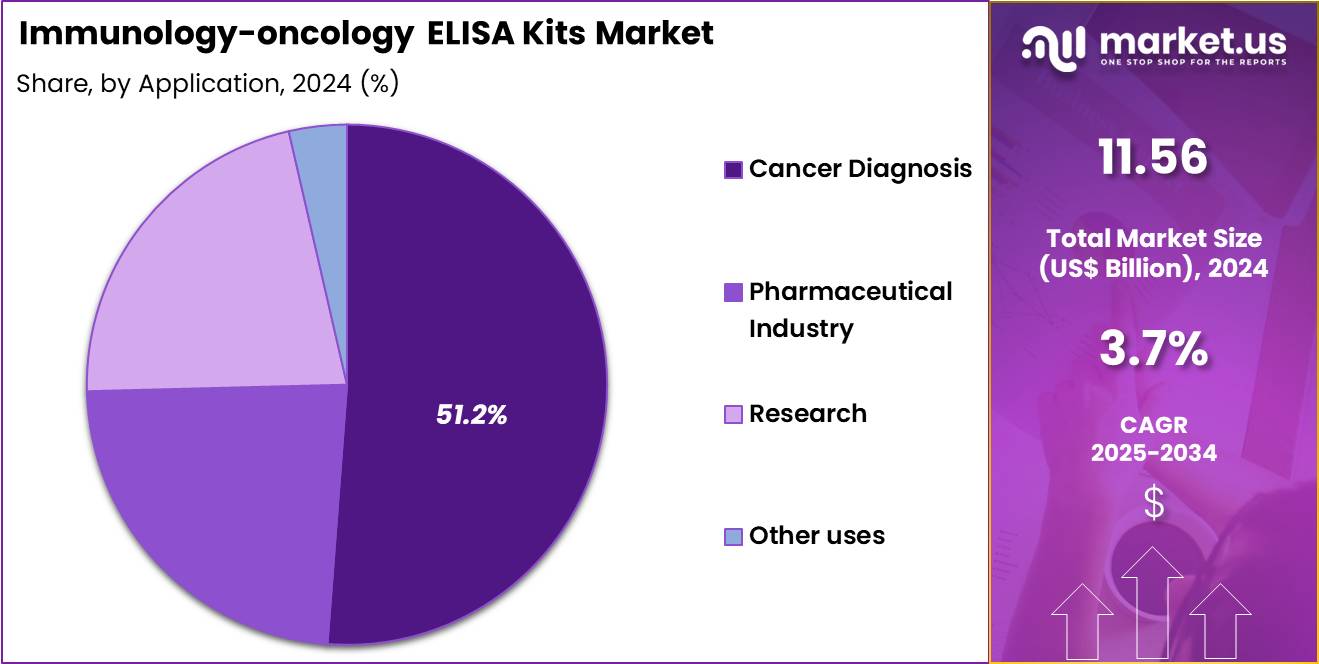
- The Application segment is divided into Cancer Diagnosis, Pharmaceutical Industry, Research, and Other uses, with Cancer Diagnosis taking the lead in 2024 with a market share of 51.2%
- The End-User segment is divided into Hospitals, Diagnostic Centers, Research Laboratories, and Others, with Hospitals taking the lead in 2024 with a market share of 42.1%
- North America led the market by securing a market share of 41.7% in 2024.
Product Type Analysis
Direct ELISA Kits accounted for an estimated 31.4% share due to their rapid workflow suited for high-throughput antigen screening. These kits are commonly used for detecting tumor-associated proteins where enzymatic labeling does not hinder antigen recognition. Their minimal reagent steps reduce variability and enable consistent assays in diagnostic laboratories.
Indirect ELISA Kits are driven by high sensitivity for detecting antibodies relevant to tumor immunology. Research laboratories prefer this format for immune profiling, cancer vaccine studies, and assessing autoantibody responses. The secondary-antibody amplification enhances signal intensity, making it suitable for low-abundance immune markers.
Application Analysis
Cancer Diagnosis leads the market with 51.2% share. ELISA tests play a central role in screening tumor markers, evaluating immune checkpoint expression, and monitoring post-treatment biomarker changes. According to Cancer.gov, an estimated 2,041,910 new cancer cases are expected to be diagnosed in the United States in 2025, while approximately 618,120 individuals are projected to die from the disease.
The leading cancer types by anticipated incidence include breast, prostate, lung and bronchus, colorectal, melanoma of the skin, bladder, kidney and renal pelvis, non-Hodgkin lymphoma, uterine corpus, pancreatic, leukemia, thyroid, and liver and intrahepatic bile duct cancers. Among men, prostate, lung, and colorectal cancers are expected to represent about 48% of all cancer diagnoses in 2025. In women, breast, lung, and colorectal cancers remain the most common, collectively accounting for an estimated 51% of all new female cancer cases in 2025.
Hospitals and oncology centers routinely utilize ELISA assays to guide immunotherapy decisions and track immune system engagement. The Pharmaceutical Industry driven by the expanding pipeline of immuno-oncology drugs and biomarker-guided clinical trials. ELISA helps in pharmacokinetic evaluation, cytokine-release assessment, drug safety studies, and companion diagnostic development.
End-User
Hospitals lead with 42.1% share due to high diagnostic volume in oncology departments. ELISA assays assist in evaluating inflammatory markers, tumor antigens, immune cell activity, and therapy-specific response biomarkers. Diagnostic Centers maintain, supported by increasing outsourcing of cancer biomarker testing.
Independent labs prefer ELISA for its cost-efficiency, reliability, and compatibility with automated analyzers. Research Laboratories are driven by extensive immuno-oncology studies, biomarker discovery projects, and translational medicine initiatives across universities and cancer institutes.
Key Market Segments
By Product Type
- Direct ELISA Kit
- Indirect ELISA Kit
- Sandwich ELISA Kit
- Competition or Inhibition
By Application
- Cancer Diagnosis
- Pharmaceutical Industry
- Research
- Other uses
By End-User
- Hospitals
- Diagnostic Centers
- Research Laboratories
- Others
Drivers
Growing Adoption of Biomarker-Driven Immuno-Oncology
Biomarker-driven immuno-oncology continues to expand as treatment decisions increasingly rely on quantifiable immune and tumor markers. According to the National Cancer Institute (NCI), over 67% of ongoing oncology clinical trials now incorporate immune biomarkers such as PD-L1, CTLA-4, tumor mutational burden (TMB), and circulating cytokines.
Biomarkers help in predicting therapeutic response, identifying non-responders early, and adjusting dosing in real time. For example, patients receiving immune checkpoint inhibitors frequently undergo ELISA-based testing for IL-6, IFN-γ, and CRP to monitor immune activation or detect cytokine release syndrome. The FDA has approved 48+ biomarker-linked cancer therapies, including PD-L1–guided treatments for lung, breast, gastric, and bladder cancers, demonstrating how biomarker quantification drives drug selection.
Moreover, large-scale programs such as the NCI’s Immuno-Oncology Translational Network and the UK’s Cancer Research UK (CRUK) initiatives emphasize integrating biomarker discovery into immunotherapy R&D. Real-world evidence also shows that biomarkers improve treatment efficiency; for instance, PD-L1 expression testing has reduced inappropriate immunotherapy prescribing by 20–30% across several US cancer centers.
Restraints
Variability in Sample Quality and Quantification
Variability in ELISA quantification remains a significant restraint, especially for low-abundance immuno-oncology biomarkers. The accuracy of ELISA depends heavily on pre-analytical handling sample type, storage, hemolysis, freeze-thaw cycles, and anticoagulants.
Studies published in the Journal of Immunological Methods show that cytokine concentrations such as IL-1β, IL-6, and TNF-α may vary by 15–40% depending solely on processing delays beyond two hours after blood draw. The NIH’s Biospecimen Research Network similarly reports a 20–50% reduction in detectable cytokines when plasma is stored at room temperature instead of being rapidly cooled or frozen.
These inconsistencies complicate multi-center clinical trials where identical samples must yield comparable results. Furthermore, heterophilic antibodies and rheumatoid factors—more common in cancer patients can falsely elevate ELISA readings, while improper plate washing may reduce sensitivity by up to 30%.
Opportunities
Expansion of Multi-Analyte Immunoassay Panels
Multi-analyte immunoassay panels are rapidly expanding as oncology shifts from single-marker interpretation to multiplex immune signatures. Modern cancer immunology recognizes that pathways such as T-cell activation, inflammation, angiogenesis, and immune suppression operate through complex cytokine networks.
As a result, multi-analyte panels that quantify 10–50 biomarkers simultaneously are gaining adoption across research institutes and clinical programs. The NIH ImmPort Database shows that over 55% of immuno-oncology studies now depend on multi-marker datasets rather than single metrics.
These panels enable more accurate profiling of tumor microenvironments by simultaneously measuring cytokines (IL-2, IL-10, IL-17), chemokines (CXCL9, CXCL10), growth factors (VEGF, TGF-β), and checkpoint molecules (PD-1, sPD-L1). In CAR-T therapy monitoring, multi-analyte panels are essential for early detection of cytokine release syndrome, where IL-6, IFN-γ, and GM-CSF levels must be tracked together. Publications from MD Anderson Cancer Center highlight that multiplex profiling increased early CRS prediction rates by 35% compared to single-marker ELISA testing.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors significantly influence the availability, pricing, and adoption of immunology-oncology ELISA kits across global diagnostic and research ecosystems. Supply chains for critical components such as monoclonal antibodies, recombinant proteins, microplates, enzymes, and specialty plastics are highly globalized. Geopolitical disruptions affecting major manufacturing hubs in the US, Europe, and East Asia can slow production and increase lead times.
For example, global freight-cost inflation between 2021 and 2023 reached up to 350% on certain routes (World Bank), directly impacting reagent import costs for diagnostic laboratories. Economic slowdowns also reshape purchasing behavior, with hospitals and academic institutes opting for mid-range ELISA kits rather than premium ultra-sensitivity options.
Geopolitical tensions influence scientific collaboration, intellectual-property movement, and cross-border shipment of biological materials. Export restrictions on laboratory enzymes and high-purity chemicals as seen in several countries during health emergencies can delay research timelines and clinical validations. Currency fluctuations further affect procurement budgets; for instance, diagnostics spending in LATAM and parts of Africa dropped during periods in which local currency depreciation increased costs of imported assays.
Latest Trends
Growing Demand for Cytokine and Checkpoint-Targeted ELISA Panels
Demand for ELISA panels targeting cytokines and immune-checkpoint markers continues to grow as immune-based therapies become standard-of-care for multiple cancers. Cytokine profiling is essential for assessing immune activation, inflammation, and toxicity.
For example, IL-6, IL-8, and IFN-γ levels are routinely quantified to evaluate immunotherapy response or detect early cytokine release syndrome (CRS). According to the FDA’s adverse event reporting data, CRS-related complications occur in 15–30% of CAR-T therapy patients, prompting hospitals to adopt rapid cytokine ELISA panels for real-time monitoring.
Checkpoint-targeted assays—such as PD-1, PD-L1, CTLA-4, TIM-3, and LAG-3 ELISA kits—are also rising sharply. PD-L1 remains one of the most clinically relevant biomarkers, with testing required or recommended across 10+ cancer types. The European Society for Medical Oncology (ESMO) reports that PD-L1 assessment influences treatment selection in over 40% of metastatic lung cancer cases.
Regional Analysis
North America is leading the Immunology-oncology ELISA Kits Market
North America represents the largest regional market share of 41.7% for immunology-oncology ELISA kits due to its advanced cancer-care infrastructure, high adoption of biomarker-driven therapies, and strong presence of biotechnology and pharmaceutical companies engaged in immunotherapy development. The region conducts a significant share of global oncology clinical trials over 45% according to ClinicalTrials.gov which drives consistent demand for cytokine, checkpoint, and tumor-marker ELISA assays used in pharmacokinetic and translational research.
The US also maintains one of the highest cancer incidence rates globally, exceeding 1.9 million new cases annually (American Cancer Society), creating sustained need for diagnostic immunoassays in hospitals and diagnostic centers. Academic institutions and research consortia such as NCI-designated cancer centers, MD Anderson, and Dana-Farber frequently rely on ELISA panels for immune-profiling and treatment-response monitoring.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is the fastest-growing region in the immunology-oncology ELISA kits market, driven by rising cancer prevalence, expanding biotechnology capabilities, and increasing investment in precision oncology. Countries such as China, Japan, and South Korea are accelerating national cancer-control programs, with China alone reporting more than 4.8 million new cancer cases annually (National Cancer Center China).
This surge fuels demand for accessible biomarker testing, including ELISA panels for cytokines, growth factors, and immune-checkpoint markers. Government-backed initiatives supporting genomic and immunotherapy research such as Japan’s Moonshot R&D and China’s Precision Medicine Initiative are boosting adoption of research-grade and clinical-grade ELISA kits.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the market include Thermo Fisher Scientific, Bio-Techne Corporation (R&D Systems), Abcam, BioLegend, BD Biosciences, MilliporeSigma (Merck KGaA), Enzo Life Sciences, RayBiotech Life, Inc., ELISAGEN Biotech, Creative Diagnostics, J&G Biotech Ltd, Elabscience and others.
Thermo Fisher provides a wide portfolio of high-sensitivity immunology-oncology ELISA kits targeting cytokines, checkpoint proteins, and tumor biomarkers. Its trusted reagents, validated antibodies, and automated platforms support clinical research, biomarker discovery, and immunotherapy development across hospitals, CROs, and academic oncology centers. Bio-Techne’s R&D Systems brand is a leading supplier of premium immunology-oncology ELISA kits known for ultra-high specificity and reproducibility.
Its assays cover cytokines, chemokines, and soluble checkpoint markers widely used in translational cancer research, immune-profiling, and preclinical immunotherapy evaluation. Abcam offers an extensive range of immunology-oncology ELISA kits designed for sensitive quantification of cytokines, immune checkpoints, and tumor-associated antigens. Its well-validated antibodies, recombinant proteins, and streamlined protocols support biomarker validation, mechanistic oncology studies, and immunotherapy response monitoring in research and diagnostic settings.
Top Key Players
- Thermo Fisher Scientific
- Bio-Techne Corporation
- Abcam
- BioLegend
- BD Biosciences
- MilliporeSigma (Merck KGaA)
- Enzo Life Sciences
- RayBiotech Life, Inc.
- ELISAGEN Biotech
- Creative Diagnostics
- J&G Biotech Ltd
- Elabscience
- Others
Recent Developments
- In April 2025 Tecan announced that it has expanded its portfolio for specialty diagnostics through an asset purchase from Cisbio Bioassays SAS. The acquisition includes certain ELISA immunoassay products four ELISA kits (two IVD kits for specialty diagnostics and disease monitoring; two research-use-only kits) indicating consolidation and reorganisation within ELISA-kit supply.
- In June 2025 Quanterix Corporation announced that its HD-X Simoa Immunoassay Analyzer received regulatory registration (Class I medical device) by the regulatory authority in South Korea (through its distribution partner). While not a classic “ELISA kit” press-release, this underscores growing regulatory acceptance for ultrasensitive immunoassay platforms which may encourage use in oncology biomarker detection, potentially stimulating demand for specialized kits.
Report Scope
Report Features Description Market Value (2024) US$ 11.85 Billion Forecast Revenue (2034) US$ 17.04 Billion CAGR (2025-2034) 3.7% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Direct ELISA Kit, Indirect ELISA Kit, Sandwich ELISA Kit, and Competition or Inhibition), By Application (Cancer Diagnosis, Pharmaceutical Industry, Research and Other uses), By End-User (Hospitals, Diagnostic Centers, Research Laboratories, and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Thermo Fisher Scientific, Bio-Techne Corporation (R&D Systems), Abcam, BioLegend, BD Biosciences, MilliporeSigma (Merck KGaA), Enzo Life Sciences, RayBiotech Life, Inc., ELISAGEN Biotech, Creative Diagnostics, J&G Biotech Ltd, Elabscience and others Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Immunology-oncology ELISA Kits MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample
Immunology-oncology ELISA Kits MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Thermo Fisher Scientific
- Bio-Techne Corporation
- Abcam
- BioLegend
- BD Biosciences
- MilliporeSigma (Merck KGaA)
- Enzo Life Sciences
- RayBiotech Life, Inc.
- ELISAGEN Biotech
- Creative Diagnostics
- J&G Biotech Ltd
- Elabscience
- Others










