Global Gene Therapy in CNS Disorder Market By Indication (Alzheimer's Disease, Huntington's Disease, Parkinson's Disease, Batten Disease) By Type (Ex-Vivo, In Vivo) By End User ( Hospitals, Speciality Clinics) Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Jan 2025
- Report ID: 136765
- Number of Pages: 352
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
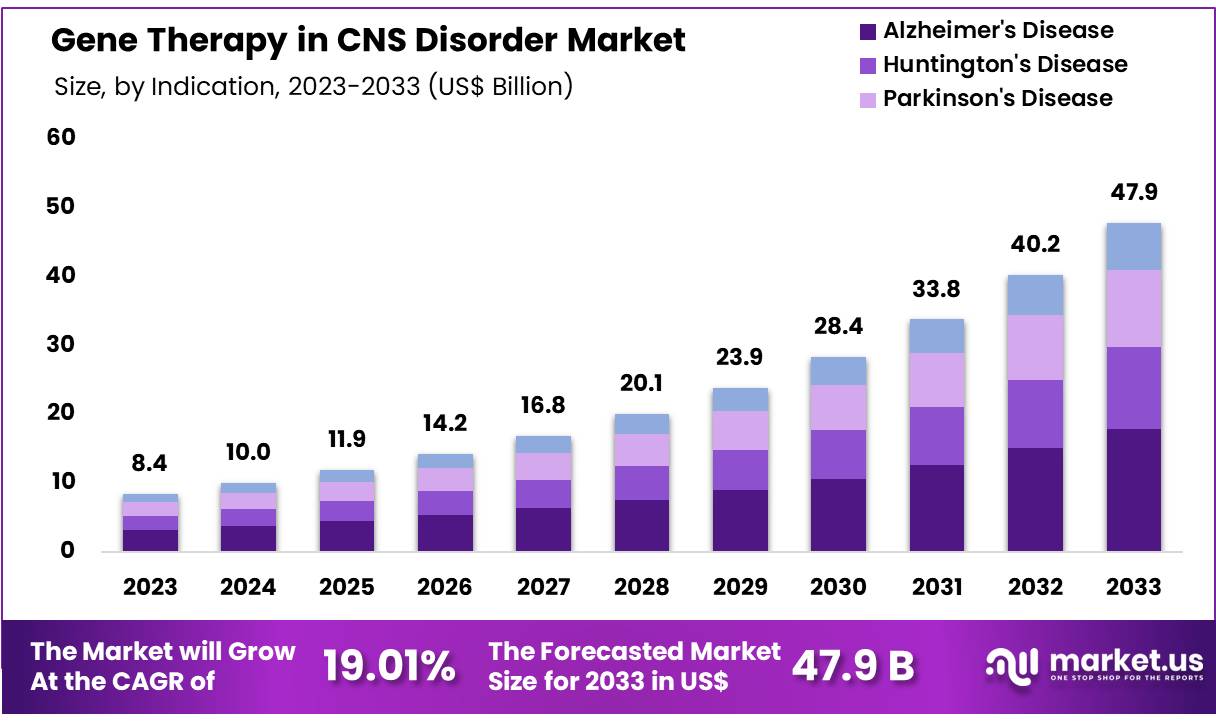
Global Gene Therapy in CNS Disorder Market size is expected to be worth around US$ 47.9 Billion by 2033 from US$ 8.4 Billion in 2023, growing at a CAGR of 19.01% during the forecast period from 2024 to 2033. With a market share over 38.30% North America held a strong lead in 2023, reaching US$ 3.2 Billion in revenue.
The gene therapy market for central nervous system (CNS) disorders is experiencing rapid growth, driven by advancements in therapeutic gene delivery targeting neurological conditions. This segment of the healthcare industry focuses on innovative strategies for managing and treating CNS disorders. Key targets of gene therapy include Parkinson’s disease, Alzheimer’s disease, Spinal Muscular Atrophy (SMA), Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS).
A major factor propelling the growth of this market is the increasing prevalence of neurological diseases worldwide. Aging populations and changing lifestyles have contributed to a rise in conditions such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and ALS. The substantial healthcare burden posed by these disorders has heightened the demand for advanced treatment options like gene therapy, which holds promise for long-term symptom management and potential reversal of disease progression.
Between 2018 and 2022, the market for gene therapy in CNS disorders demonstrated significant growth, reflecting a 19% increase in value. This growth was supported by rising awareness among healthcare professionals and patients, technological advancements in gene therapy, and favorable regulatory frameworks.
Additionally, increased investments in research and development have facilitated the creation of novel therapeutic approaches for disorders such as Parkinson’s disease, SMA, and ALS. These factors have solidified the market’s position as a promising frontier in neurological treatment.
Key Takeaways
- Market Size: Global Gene Therapy in CNS Disorder Market size is expected to be worth around US$ 47.9 Billion by 2033 from US$ 8.4 Billion in 2023.
- Market Growth: The market growing at a CAGR of 19.01% during the forecast period from 2024 to 2033.
- Indication Analysis: The Gene Therapy in CNS Disorder market is segmented by indications, with Alzheimer’s disease emerging as the dominant segment, holding 37.4% of the market share in 2023.
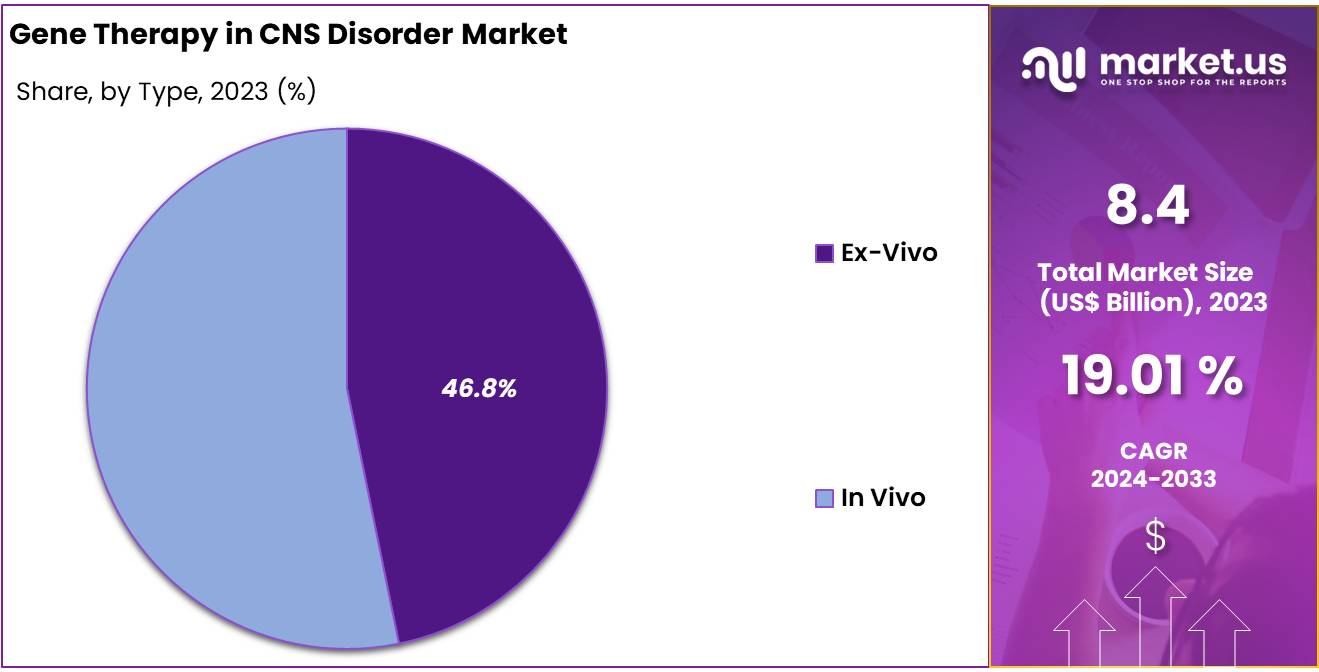
- Type Analysis: The Gene Therapy in CNS Disorder market is segmented by type into In Vivo and Ex Vivo approaches, with In Vivo gene therapy dominating, holding a significant 53.2% market share in 2023.
- End-Use Analysis: The Gene Therapy in CNS Disorder market is segmented by end-users, with hospitals accounting for a dominant 64.9% market share in 2023.
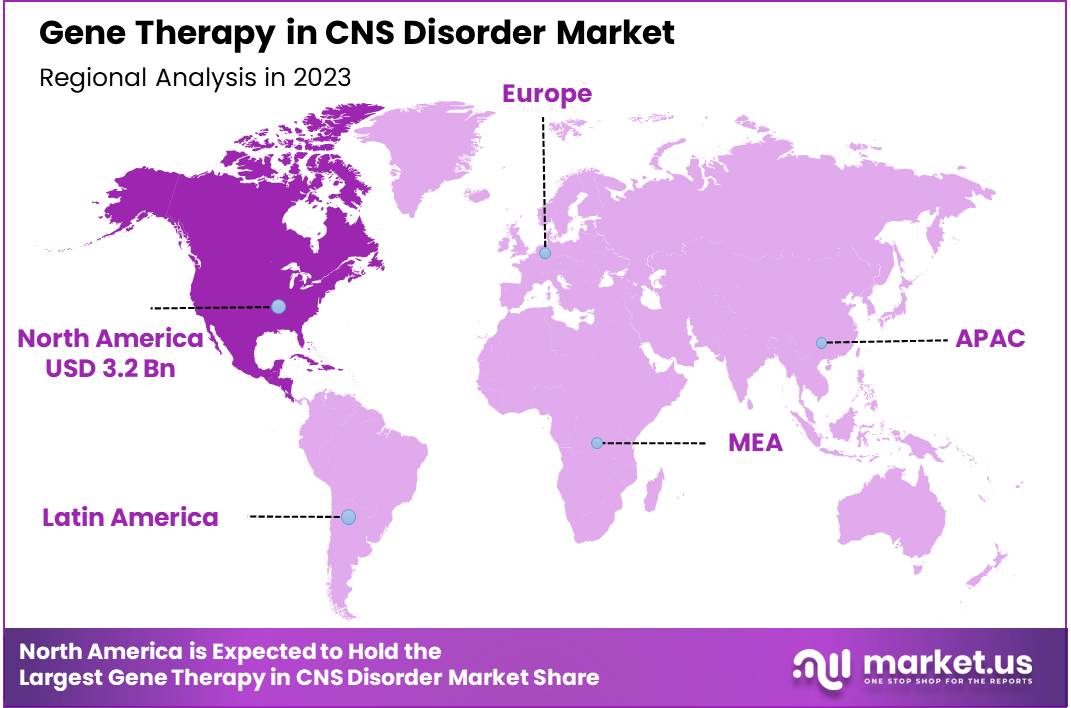
- Regional Analysis: North America is anticipated to acquire a market share of about 38.30% in the forecast period.
Indication Analysis
The Gene Therapy in CNS Disorder market is segmented by indications, with Alzheimer’s disease emerging as the dominant segment, holding 37.4% of the market share in 2023. The high prevalence of Alzheimer’s disease, coupled with advancements in targeted gene delivery systems, has driven substantial research and investment in this segment. Increasing global cases due to aging populations and the lack of curative treatments further bolster its dominance.
Huntington’s disease is another significant segment, benefiting from advancements in gene-editing technologies such as CRISPR. Efforts to address this genetic disorder have propelled its growth.
The Parkinson’s disease segment is also witnessing substantial growth due to the increasing burden of the disease and the promise of gene therapy in addressing its underlying causes. Gene therapies aimed at restoring dopamine function are particularly noteworthy.
Batten disease, a rarer neurological condition, represents a growing niche, driven by increasing clinical trials and orphan drug designations for gene therapy applications.
Type Analysis
The Gene Therapy in CNS Disorder market is segmented by type into In Vivo and Ex Vivo approaches, with In Vivo gene therapy dominating, holding a significant 53.2% market share in 2023. The preference for In Vivo therapies stems from their ability to deliver genetic material directly into the patient’s brain or spinal cord using viral vectors, ensuring targeted and efficient treatment. This method is particularly advantageous for CNS disorders where direct access to affected regions is critical for therapeutic efficacy.
Ex Vivo gene therapy, while less dominant, is growing steadily due to its unique advantages. This approach involves modifying a patient’s cells outside the body and reintroducing them to address CNS disorders. Ex Vivo techniques are increasingly used in research and clinical trials, particularly for disorders like Huntington’s disease and Batten disease, where specific cellular modifications can yield promising results.
End User Analysis
The Gene Therapy in CNS Disorder market is segmented by end-users, with hospitals accounting for a dominant 64.9% market share in 2023. Hospitals serve as primary treatment centers for CNS disorders due to their advanced infrastructure, specialized equipment, and access to multidisciplinary teams. The availability of cutting-edge gene therapy procedures and trained professionals makes hospitals the preferred choice for patients requiring complex treatments for conditions such as Alzheimer’s disease, Parkinson’s disease, and ALS.
Specialty clinics represent a growing segment in the market. These clinics focus on providing dedicated care for specific neurological conditions, offering a personalized approach that appeals to patients seeking targeted treatments. Specialty clinics often collaborate with research institutions and pharmaceutical companies, driving innovation in gene therapy applications for rare CNS disorders such as Batten disease and Huntington’s disease.
While hospitals maintain their dominance, the increasing presence and accessibility of specialty clinics are expected to contribute significantly to market growth in the coming years.
Key Market Segments
Indication
- Alzheimer’s Disease
- Huntington’s Disease
- Parkinson’s Disease
- Batten Disease
Type
- Ex-Vivo
- In Vivo
End User
- Hospitals
- Speciality Clinics
Driver
Increasing Prevalence of CNS Disorders
The rising prevalence of central nervous system (CNS) disorders, including Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease, is a significant driver of the gene therapy market. According to the World Health Organization (WHO), neurological disorders are among the leading causes of disability and death globally, affecting millions annually.Gene therapy offers the potential to address these disorders at their root cause by modifying faulty genes. Advances in genomic research and technologies such as CRISPR-Cas9 have further facilitated the development of precise therapies, enhancing their effectiveness.
Additionally, growing awareness among healthcare professionals and patients about the potential of gene therapy has boosted its adoption. Regulatory bodies, including the FDA and EMA, have streamlined the approval pathways for innovative gene therapies, making it easier for companies to bring products to market. This confluence of factors is driving substantial growth in the gene therapy market for CNS disorders.
Trend
Rising Investment in Gene Therapy Research
A key trend shaping the CNS gene therapy market is the surge in investments by pharmaceutical and biotechnology companies. The market has seen substantial funding for research and development (R&D) aimed at creating effective gene therapies for previously untreatable CNS disorders.For example, companies are leveraging advanced delivery systems such as adeno-associated viral (AAV) vectors to enhance the precision and safety of therapies. Collaborations between academic institutions, government agencies, and private enterprises have also accelerated innovation.
Notably, gene therapy start-ups and specialized firms are increasingly partnering with larger pharmaceutical companies to expand their research capabilities and commercialization potential. Clinical trials targeting CNS disorders have increased, with several products entering late-stage trials or receiving regulatory approval. This growing focus on innovation has led to a steady pipeline of novel gene therapy products, positioning the market as a hub of groundbreaking advancements in neurological healthcare.
Restraint
High Costs of Gene Therapy
One major restraint in the gene therapy market for CNS disorders is the high cost associated with developing and administering these therapies. Gene therapies often involve complex manufacturing processes, including the production of viral vectors and precision genetic modifications, which contribute to their high price tags. For instance, the average cost of gene therapy can exceed $1 million per patient, making it inaccessible for many individuals and healthcare systems, particularly in low- and middle-income countries.Moreover, the lack of widespread reimbursement policies further limits the availability of these therapies. While some governments and insurers have introduced payment models, such as outcomes-based reimbursement, these are not yet universally adopted. Additionally, the high costs pose challenges for smaller biotechnology firms, which may struggle to finance extensive clinical trials or regulatory approvals. These financial barriers hinder the broader adoption of gene therapies for CNS disorders.
Opportunity
Expanding Applications of Gene Therapy
The gene therapy market for CNS disorders presents significant opportunities, particularly in expanding its applications to rare and orphan diseases. With advancements in genetic research and sequencing technologies, researchers are uncovering the genetic underpinnings of numerous CNS conditions, many of which currently lack effective treatments. Governments and regulatory bodies have introduced incentives, such as orphan drug designations and expedited approval pathways, to encourage innovation in this area.Moreover, the development of next-generation delivery systems, including non-viral vectors and nanoparticles, is expected to enhance therapy efficacy and safety. Emerging markets in Asia-Pacific, Latin America, and the Middle East also offer untapped potential due to growing healthcare infrastructure and increasing awareness of gene therapies.
Collaborations between global pharmaceutical companies and regional healthcare providers could further expand market access. These opportunities position gene therapy as a transformative solution for a wide range of CNS disorders.
Regional Analysis
North America is anticipated to acquire a market share of about 38.30% in the forecast period. This growth is attributable to the high prevalence of CNS disorders in North America, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, which has led to a strong demand for new treatments. Additionally, North America has a well-developed healthcare infrastructure, along with a high level of research and development, significant investment, and a supportive regulatory environment.
The United States of America, in particular, has been experiencing an increase in CNS disorders related deaths, which may drive demand for treatment. The United States of America holds the highest share in the North American market, followed by Canada.
Presence of a large number of pharmaceutical companies, which are investing heavily in the development of new, targeted treatments for the condition, and high level of healthcare expenditure leading to a large patient population are some of the factors responsible for the growth of the market in the region.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The Gene Therapy in CNS Disorder market is characterized by the presence of prominent players driving advancements in treatment solutions. These organizations are heavily investing in research and development to enhance gene therapy technologies, focusing on viral vectors and gene editing techniques to target neurological disorders effectively. Partnerships with academic institutions and healthcare providers are fostering innovation and accelerating clinical trials for conditions like Alzheimer’s, Parkinson’s, and Huntington’s diseases.
Key players are expanding their portfolios through strategic collaborations, acquisitions, and licensing agreements to strengthen their market position. Significant funding is being directed toward developing innovative therapies with regulatory approvals and orphan drug designations for rare CNS disorders. The continuous focus on expanding treatment accessibility and addressing unmet clinical needs positions these companies as pivotal in shaping the market’s growth trajectory.
Market Key Players
- Voyager Therapeutics
- Spark Therapeutics
- Novartis AG
- Bluebird Bio Inc.
- Biogen
- Pfizer Inc.
- Rapa Therapeutics
- BrainStorm Cell Therapeutics
- Eli Lily and Company
- UniQure Biopharma
Recent Developments
- Spark Therapeutics, July 2025: In a strategic move to enhance its gene therapy portfolio, Spark Therapeutics completed the acquisition of a biotech firm specializing in Alzheimer’s research. This acquisition, finalized in July 2025, is expected to leverage synergistic capabilities in gene therapy technologies to accelerate development in Alzheimer’s disease treatments.
- Novartis AG, September 2025: Novartis AG launched an innovative gene therapy for Huntington’s disease, which received regulatory approval in September 2025. This therapy, based on cutting-edge genetic editing tools, aims to significantly slow disease progression, marking a major advancement in treatment options for Huntington’s disease.
- Bluebird Bio Inc., October 2025: Bluebird Bio Inc. introduced a new ex vivo gene therapy for Spinal Muscular Atrophy (SMA) in October 2025. This therapy involves modifying patient stem cells outside the body to produce proteins essential for muscle development and function, providing a vital treatment alternative for SMA patients.
- Biogen, November 2025: Biogen expanded its CNS disorder treatment range with a novel gene therapy for ALS, launched in November 2025. The therapy focuses on delivering genetic material directly to motor neurons, offering hope for ALS patients through potentially improved motor functions and slowed disease progression.
- Pfizer Inc., December 2025: Pfizer Inc. announced a merger with a leading neurological research institute in December 2025, aiming to bolster its research capabilities in gene therapies for CNS disorders. This merger is set to enhance Pfizer’s resources and expertise in developing next-generation treatments for diseases like Alzheimer’s and Parkinson’s.
Report Scope
Report Features Description Market Value (2023) US$ 8.4 Billion Forecast Revenue (2033) US$ 47.9 Billion CAGR (2024-2033) 19.01 Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Indication (Alzheimer’s Disease, Huntington’s Disease, Parkinson’s Disease, Batten Disease) By Type (Ex-Vivo, In Vivo) By End User ( Hospitals, Speciality Clinics) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Voyager Therapeutics, Spark Therapeutics, Novartis AG, Bluebird Bio Inc., Biogen, Pfizer Inc., Rapa Therapeutics, BrainStorm Cell Therapeutics, Eli Lily and Company, UniQure Biopharma Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Gene Therapy in CNS Disorder MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample
Gene Therapy in CNS Disorder MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Voyager Therapeutics
- Spark Therapeutics
- Novartis AG
- Bluebird Bio Inc.
- Biogen
- Pfizer Inc.
- Rapa Therapeutics
- BrainStorm Cell Therapeutics
- Eli Lily and Company
- UniQure Biopharma










