Global MSI-H/dMMR Market By Treatment Type (Immune Checkpoint Inhibitors (ICIs) and Combination Therapy), By Stage (Early Stages (Stage I and II) and Advanced Stages (Stage III and IV)), By Indication (Endometrial, Gastric, Colorectal Cancer, Small Intestine Cancer, Cervical Cancer and Others), By End-User (Hospitals, Oncology Clinics, Research Institutions, and Homecare), By Region, and Key Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: March 2025
- Report ID: 140940
- Number of Pages: 200
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
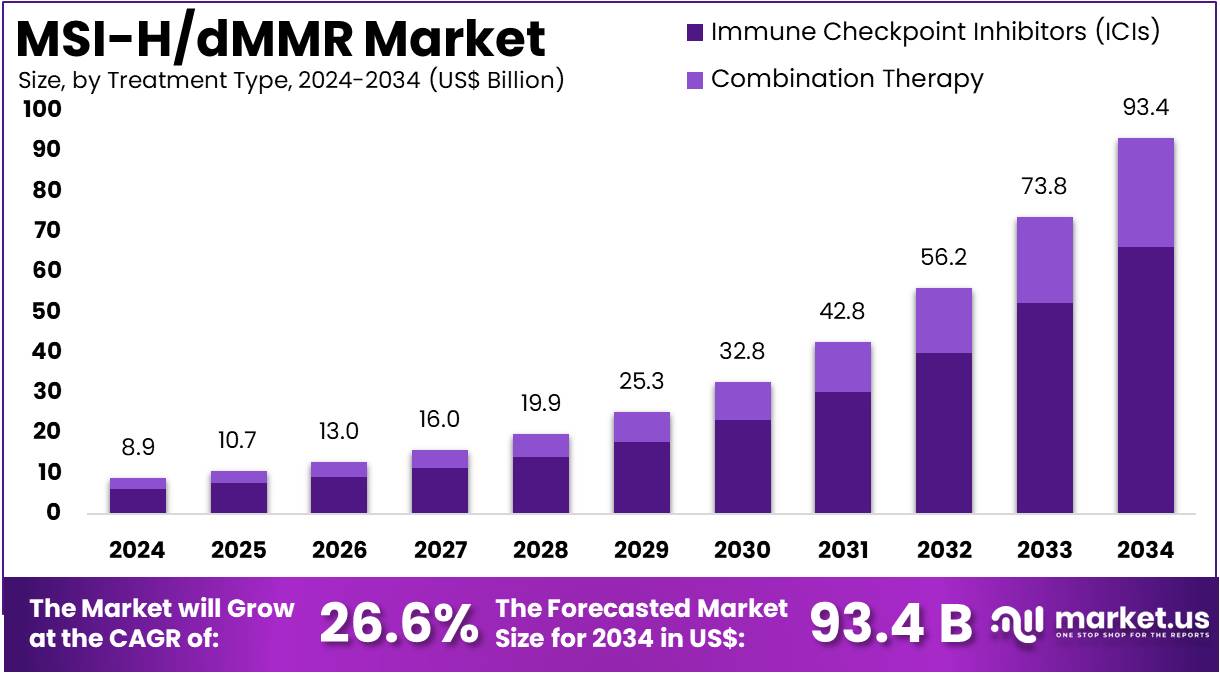
The Global MSI-H/dMMR Market Size is expected to be worth around US$ 93.4 Billion by 2034, from US$ 8.9 Billion in 2024, growing at a CAGR of 26.6% during the forecast period from 2025 to 2034. North America held a dominant market position, capturing more than a 53.1% share and holds US$ 4.7 Billion market value for the year.
The global MSI-H/dMMR market is driven by the growing prevalence of MSI-H/dMMR cancers, particularly in colorectal, endometrial, and gastric cancers, where this genetic anomaly is a key biomarker for targeted therapies. Advances in precision medicine and the increasing adoption of immunotherapies, such as immune checkpoint inhibitors, have significantly contributed to market growth, offering promising treatments for patients with MSI-H/dMMR cancers.
Additionally, the expanding pipeline of drugs designed to specifically target MSI-H/dMMR tumors is further boosting market prospects. The rise in cancer awareness, improved diagnostic techniques, and personalized treatment options are also fueling market expansion. However, challenges such as high treatment costs, limited availability of specialized tests, and regulatory hurdles may impact market growth.
On the other hand, ongoing research and clinical trials to explore new therapeutic strategies and improve patient outcomes are expected to offer significant opportunities for growth in the coming years.
- In 2020, the PMDA approved nivolumab for the treatment of MSI-H/dMMR colorectal cancer as well, making it one of the first checkpoint inhibitors authorized for this indication. This approval was based on the CheckMate-142 trial, which showed that nivolumab significantly improved survival rates in patients with MSI-H/dMMR colorectal cancer.
MSI-H/dMMR Market, Global Analysis, 2020-2024 (US$ Billion)
Global 2020 2021 2022 2023 2024 CAGR Revenue 6.0 6.2 6.6 7.6 8.9 26.6% Key Takeaways
- The global MSI-H/dMMR market was valued at USD 8.8 billion in 2024 and is anticipated to register substantial growth of USD 97.3 billion by 2034, with 26.6% CAGR.
- In 2024, the immune checkpoint inhibitors (ICIs) segment took the lead in the global market, securing 71.0% of the total revenue share.
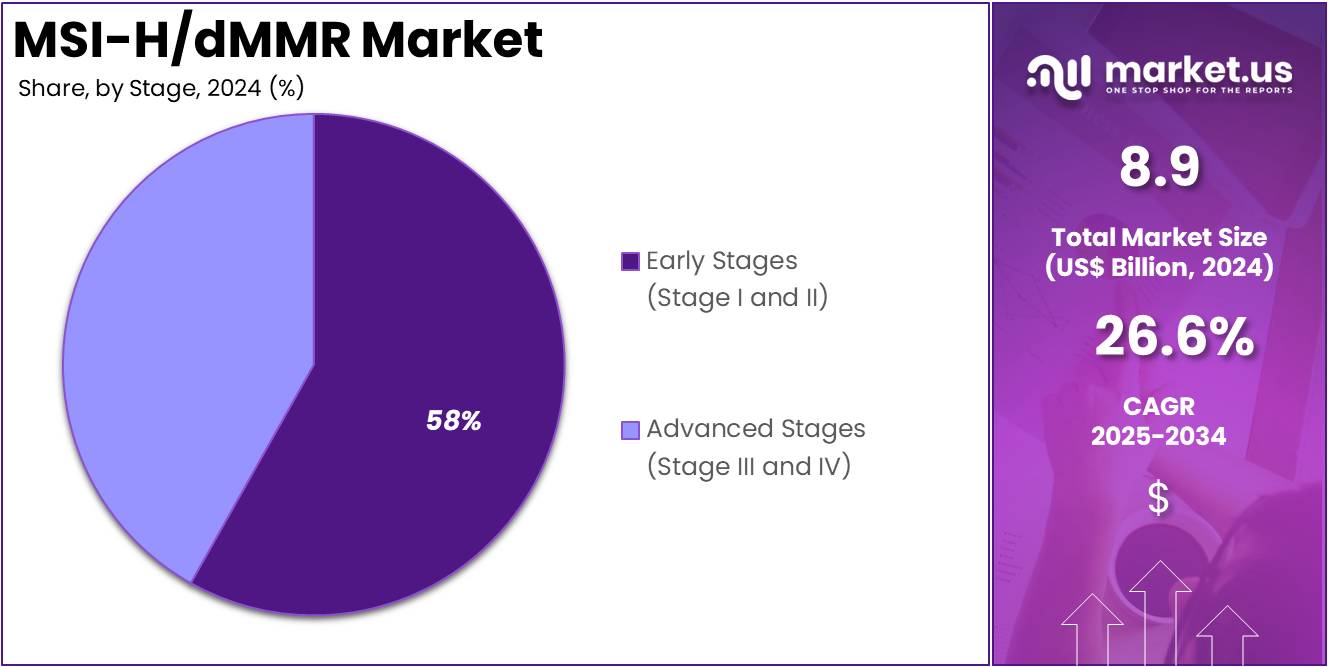
- The early stages (Stage I and II) segment took the lead in the global market, securing 58.4% of the total revenue share.
- The endometrial segment took the lead in the global market, securing 24.1% of the total revenue share.
- The hospitals segment took the lead in the global market, securing 54.0% of the total revenue share.
- North America maintained its leading position in the global market with a share of over 53.1% of the total revenue.
Treatment Type Analysis
Based on treatment type the market is fragmented into immune checkpoint inhibitors (ICIs) and combination therapy. Amongst these, immune checkpoint inhibitors (ICIs) segment dominated the global MSI-H/dMMR market capturing a significant market share of 71.0% in 2024. Immune checkpoint inhibitors (ICIs) play a crucial role in treating MSI-H/dMMR cancers. These cancers have a high number of mutations, which can make them more visible to the immune system.
However, they often evade immune detection using checkpoint proteins like PD-1/PD-L1. Checkpoint inhibitors, such as pembrolizumab and nivolumab, block these proteins, allowing the immune system to recognize and attack cancer cells more effectively. As MSI-H/dMMR tumors are more “immunogenic” (they have more mutations), they tend to respond well to these treatments, offering a promising option for patients with cancers like colorectal or endometrial cancer.
MSI-H/dMMR, Treatment Type Analysis, 2020-2024 (US$ Billion)
Treatment Type 2020 2021 2022 2023 2024 Immune Checkpoint Inhibitors (ICIs) 4.2 4.3 4.7 5.4 6.3 Combination therapy 1.8 1.8 1.9 2.2 2.6 Stage Analysis
The market is fragmented by stage into early stages (Stage I and II) and advanced stages (Stage III and IV). Early stages (Stage I and II) dominated the global MSI-H/dMMR market capturing a significant market share of 58.4% in 2024. The early stages (Stage I and II) of MSI-H/dMMR cancers represented a critical segmental share in the global market, characterized by unique diagnostic and therapeutic approaches aimed at improving patient outcomes.
MSI-H (Microsatellite Instability-High) and dMMR (Deficient Mismatch Repair) biomarkers are frequently observed in various cancers, including colorectal, endometrial, and gastric cancers. These stages emphasize early detection and tailored treatment strategies to maximize efficacy and long-term benefits.
- According to National Institute of Health, approximately 5% to 15% of colorectal cancers (CRC) exhibit deficiencies in the mismatch repair (dMMR) system and high microsatellite instability (MSI-H). These dMMR/MSI-H tumors are marked by a defective DNA repair mechanism, making their immune microenvironment more responsive to immunotherapy.
- Furthermore, MSI-high was identified in 17% (17 out of 100) of endometrioid-type adenocarcinomas, but was not detected in any of the non-endometrioid carcinomas (0 out of 9).
MSI-H/dMMR, Stage Analysis, 2020-2024 (US$ Billion)
Stage 2020 2021 2022 2023 2024 Early Stages (Stage I and II) 3.5 3.6 3.8 4.4 5.2 Advanced Stages (Stage III and IV) 2.5 2.6 2.8 3.2 3.7 Indication Analysis
The market is fragmented by indication into endometrial, gastric, colorectal cancer, small intestine cancer, cervical cancer and others. Endometrial dominated the global MSI-H/dMMR market capturing a significant market share of 24.1% in 2024. Endometrial cancer, specifically microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) subtypes, constitutes a dominant segment in the Global MSI-H/dMMR Market.
MSI-H/dMMR tumors are characterized by genetic instability due to defects in the DNA mismatch repair system, leading to high mutational loads. These mutations are prevalent in endometrial cancers, with approximately 20-30% of all endometrial cancer cases exhibiting MSI-H/dMMR markers, positioning this indication as a key focus area in immunotherapy and precision oncology.
End-User Analysis
The market is fragmented by end-user into hospitals, oncology clinics, research institutions, and homecare. Hospitals dominated the global MSI-H/dMMR market capturing a significant market share of 54.0% in 2024. This dominant position is largely driven by the comprehensive care and advanced treatment capabilities that hospitals offer, which are essential for patients undergoing complex immuno-oncology therapies.
One key factor contributing to the growth of the hospital segment is the advanced-stage nature of cancers treated with immuno-oncology therapies, particularly those with MSI-H and dMMR characteristics. Patients diagnosed with colorectal, endometrial, and gastric cancers, which are among the most common cancers associated with MSI-H/dMMR, often require aggressive treatment regimens.
Hospitals are the primary institutions equipped with the infrastructure to handle such advanced-stage cancers, including complex treatments like immune checkpoint inhibitors (ICIs), such as Pembrolizumab (Keytruda) and Nivolumab (Opdivo), which are often administered via infusion and require careful monitoring for side effects.
MSI-H/dMMR, Indication Analysis, 2020-2024 (US$ Billion)
Indication 2020 2021 2022 2023 2024 Endometrial 1.4 1.4 1.5 1.8 2.1 Gastric 1.2 1.2 1.3 1.4 1.7 Colorectal Cancer 1.3 1.3 1.4 1.7 2.0 Small Intestine cancer 0.9 1.0 1.0 1.1 1.3 Cervical Cancer 0.8 0.8 0.8 0.9 1.1 Others 0.4 0.5 0.5 0.6 0.7 Key Segments Analysis
By Treatment Type
- Immune Checkpoint Inhibitors (ICIs)
- Pembrolizumab (Keytruda)
- Nivolumab (Opdivo)
- Dostarlimab (Jemperli)
- Ipilimumab (Yervoy)
- Combination Therapy
- o ICI+Chemotherapy
- o Others
By Stage
- Early Stages (Stage I and II)
- Advanced Stages (Stage III and IV)
By Indication
- Endometrial
- Gastric
- Colorectal Cancer
- Small Intestine Cancer
- Cervical Cancer
- Others
By End User
- Hospitals
- Oncology Clinics
- Research Institutions
- Homecare
Drivers
Increasing Prevalence of Cancer Cases Worldwide
The increasing prevalence of cancer cases worldwide is a major driver of growth in the global MSI-H/dMMR market. MSI-H/dMMR tumors, commonly found in cancers such as colorectal, endometrial, and gastric cancers, are gaining attention due to their association with poor prognosis and the potential for targeted treatments.
As cancer rates rise globally, particularly in developed and developing countries, the demand for personalized and effective therapies, such as immune checkpoint inhibitors, has escalated. MSI-H/dMMR testing has become a key tool in identifying suitable candidates for these advanced treatments, thereby improving patient outcomes.
- According to WHO, there were 19.3 billion new cancer cases and 9.6 billion cancer deaths worldwide in 2020. The most common cancers globally include lung cancer, breast cancer, colorectal cancer, prostate cancer, and stomach cancer.
- WHO forecasts that the global number of cancer cases will rise to 30 billion by 2040, driven largely by an aging population and increasing risk factors.
Restraints
Lack of Insurance Coverage
The high cost of MSI-H/dMMR testing and therapies, coupled with a lack of expertise in emerging markets, poses significant challenges to the growth of the global MSI-H/dMMR market. Advanced treatments for MSI-H/dMMR cancers, such as immune checkpoint inhibitors, are often expensive, limiting accessibility, particularly in low- and middle-income countries.
This financial barrier prevents many patients from benefiting from precision medicine, which is crucial for treating MSI-H/dMMR-positive tumors effectively. Additionally, a shortage of trained healthcare professionals and specialized laboratories in emerging markets further exacerbates the problem. These regions may lack the necessary infrastructure to perform accurate diagnostics, slowing the adoption of MSI-H/dMMR testing and treatment.
Opportunities
Growing Drug Approvals
The growing number of drug approvals for MSI-H/dMMR cancers is creating significant growth opportunities for the global MSI-H/dMMR market. As research advances, several immune checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), have received approval for treating MSI-H/dMMR-positive tumors, particularly in cancers like colorectal, endometrial, and gastric cancers.
These approvals have opened new therapeutic avenues, increasing the demand for MSI-H/dMMR testing to identify patients eligible for such treatments. The expanding pipeline of drugs targeting MSI-H/dMMR cancers is expected to further accelerate this trend, offering innovative solutions for previously hard-to-treat patients.
With regulatory bodies like the FDA and EMA continuing to fast-track approvals for targeted therapies, more options are becoming available for personalized cancer treatment. This influx of approved drugs enhances treatment efficacy, improves patient outcomes, and ultimately drives the adoption of MSI-H/dMMR-targeted therapies, creating a dynamic and rapidly expanding market.
- For instance, in April 2022, the European Commission granted approval to Merck’s KEYTRUDA. This anti-PD-1 therapy is now authorized as a monotherapy for treating specific cancer types. It targets adults with colorectal cancer that is either unresectable or metastatic, featuring microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR). This approval follows previous treatments with fluoropyrimidine-based combinations. KEYTRUDA is also approved for treating advanced or recurrent endometrial carcinoma.
Impact of macroeconomic factors / Geopolitical factors
Macroeconomic and geopolitical factors significantly impact the global MSI-H/dMMR market. Economic downturns or financial instability can limit healthcare budgets, particularly in emerging markets, affecting the affordability and accessibility of MSI-H/dMMR testing and treatments. High costs associated with advanced therapies and diagnostics may become even more prohibitive during economic challenges, leading to slower adoption and limited market growth in these regions.
On the geopolitical front, trade barriers, regulatory changes, or conflicts can disrupt the supply chains for essential pharmaceutical products, delaying the availability of MSI-H/dMMR-targeted therapies in certain markets. Additionally, political uncertainty can lead to shifts in healthcare priorities, potentially reducing investment in cancer treatment innovations.
Conversely, geopolitical stability and strong economic performance in developed markets can foster increased research funding, accelerate drug approvals, and expand healthcare access, supporting market growth. Thus, global economic and political conditions play a pivotal role in shaping the dynamics of the MSI-H/dMMR market.
Trends
The global MSI-H/dMMR market is witnessing several key trends that are shaping its growth. One major trend is the increasing adoption of immunotherapy, particularly immune checkpoint inhibitors, for treating MSI-H/dMMR-positive cancers. Drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) have shown promising results, leading to more frequent approvals and expanded indications.
Another trend is the rising focus on personalized medicine, with more emphasis on genetic testing to identify MSI-H/dMMR tumors, enabling tailored therapies for better patient outcomes. Additionally, advancements in diagnostic technologies are making MSI-H/dMMR testing more accessible, precise, and affordable, which is driving wider adoption.
There’s also a growing interest in combination therapies, where MSI-H/dMMR-targeted treatments are being paired with other forms of cancer therapy to improve efficacy. Furthermore, the expanding research pipeline, with new therapies in clinical trials, is fueling optimism for more treatment options. These trends are collectively propelling the growth and evolution of the MSI-H/dMMR market.
Regional Analysis
In 2024, North America dominated the market, holding over 53.1% of the market share with a value of US$ 4.7 billion. North America holds a significant position in the global MSI-H/dMMR market due to several key factors. The region, particularly the United States, has a well-established healthcare infrastructure with access to cutting-edge diagnostics and therapies. This enables the rapid adoption of advanced treatments, such as immune checkpoint inhibitors, which are commonly used for MSI-H/dMMR-positive cancers.
Additionally, North America is home to major pharmaceutical companies driving innovation and the approval of new drugs targeting MSI-H/dMMR tumors. Strong regulatory support from agencies like the FDA has accelerated the approval process for MSI-H/dMMR-targeted therapies, fostering growth in the market. Moreover, rising awareness and demand for personalized cancer treatments are contributing to the expansion of the market in the region.
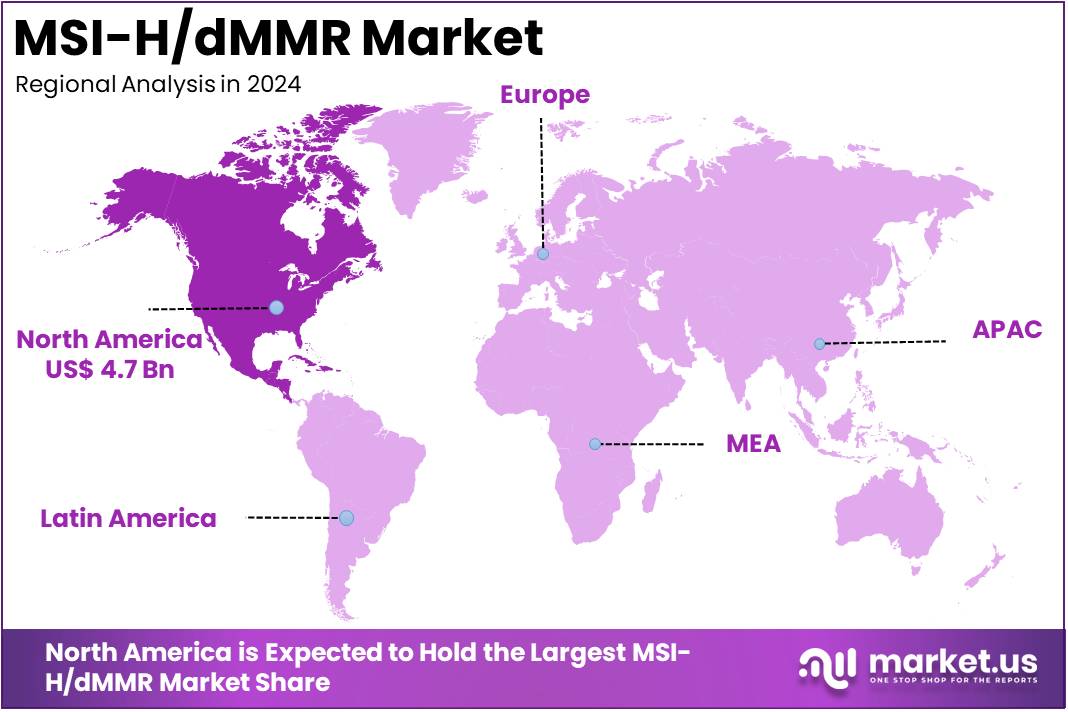
MSI-H/dMMR Market, Country/Regional Analysis, 2020-2024 (US$ Billion)
Country/Region 2020 2021 2022 2023 2024 U.S. 2.5 2.6 2.8 3.1 3.6 Europe 1.6 1.6 1.7 2.0 2.3 Japan 0.6 0.6 0.6 0.7 0.8 Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The competitive landscape of the MSI-H/dMMR market is marked by the dominance of large pharmaceutical players such as Merck, Bristol-Myers Squibb, and Roche, who are leveraging their immune checkpoint inhibitors to target MSI-H/dMMR cancers.
However, competition is intensifying as smaller biotech companies and innovative therapies such as CAR-T cell therapy, neoantigen vaccines, and oncolytic viruses enter the market. With expanding indications and ongoing clinical trials, the landscape is expected to evolve rapidly, with combination therapies and personalized treatments becoming central strategies to address unmet needs in MSI-H/dMMR cancers.
Top Key Players in the MSI-H/dMMR Market
- Pfizer Inc.
- Merck & Co.
- AstraZeneca
- F. Hoffmann-La Roche Ltd
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- GSK plc.
- BeiGene LTD.
- Summit Therapeutics Inc.
- Sanofi
- Novartis AG
- AbbVie Inc.
- Amgen Inc.
- Johnson & Johnson Services, Inc.
Recent Developments
- In March 2020, The KEYNOTE-177 trial, demonstrated that Pembrolizumab (Keytruda) significantly improved progression-free survival in patients with MSI-H colorectal cancer compared to standard chemotherapy. The trial showed that PFS was nearly doubled, with 12.5 months of median progression-free survival in the Pembrolizumab group compared to 7.1 months in the chemotherapy group. These studies underscore the significant role that advancements in targeted immunotherapy, particularly PD-1 inhibitors, are playing in reshaping the treatment landscape for MSI-H dMMR cancers.
- In April 2022, Merck announced that the European Commission has approved KEYTRUDA, Merck’s anti-PD-1 therapy, as monotherapy for the treatment of microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) tumors in adults with unresectable or metastatic colorectal cancer after previous fluoropyrimidine-based combination therapy; advanced or recurrent endometrial carcinoma.
Report Scope
Report Features Description Market Value (2024) US$ 8.9 billion Forecast Revenue (2034) US$ 93.4 billion CAGR (2025-2034) 26.6% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Treatment Type (Immune Checkpoint Inhibitors (ICIs) and Combination Therapy), By Stage (Early Stages (Stage I and II) and Advanced Stages (Stage III and IV)), By Indication (Endometrial, Gastric, Colorectal Cancer, Small Intestine Cancer, Cervical Cancer and Others), By End-User (Hospitals, Oncology Clinics, Research Institutions, and Homecare) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Pfizer Inc., Merck & Co., AstraZeneca, F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Eli Lilly and Company, GSK plc., BeiGene LTD., Summit Therapeutics Inc., Sanofi, Novartis AG, AbbVie Inc., Amgen Inc., and Johnson & Johnson Services, Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) 
-
-
- Pfizer Inc.
- Merck & Co.
- AstraZeneca
- F. Hoffmann-La Roche Ltd
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- GSK plc.
- BeiGene LTD.
- Summit Therapeutics Inc.
- Sanofi
- Novartis AG
- AbbVie Inc.
- Amgen Inc.
- Johnson & Johnson Services, Inc.










