Clinical Trial Investigative Site Network Market By Therapeutic Area (Pain Management, Oncology, Cardiology, and Others), By Phase (Phase IV, Phase III, Phase II, and Phase I), By End-use (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Dec 2024
- Report ID: 135638
- Number of Pages: 244
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
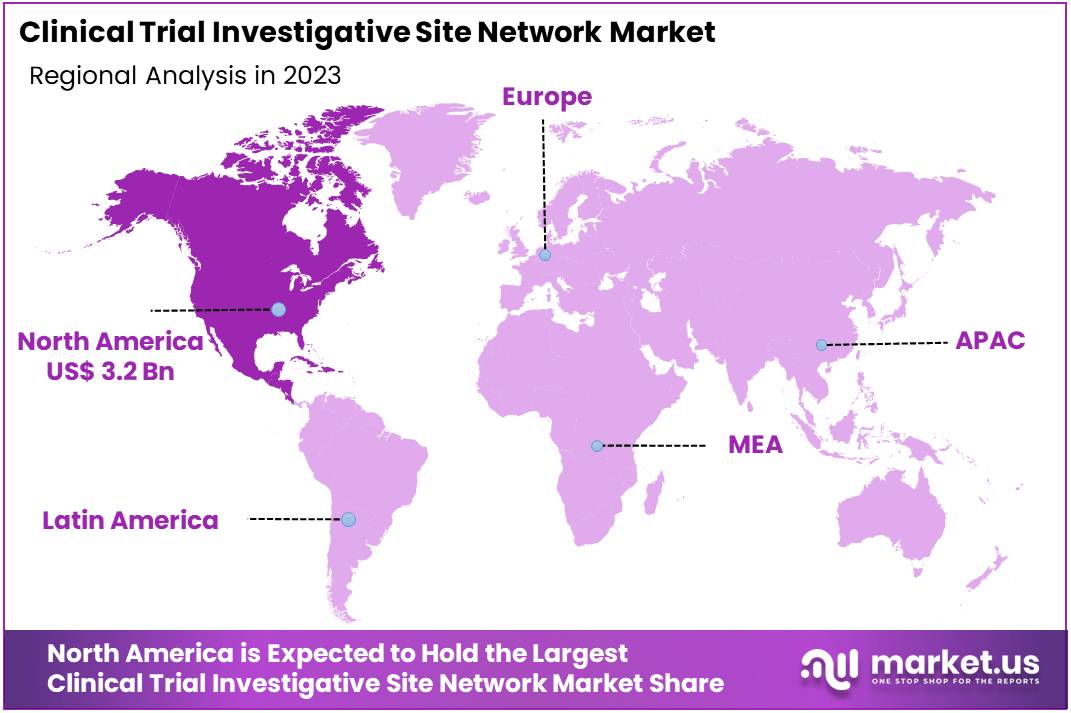
The Global Clinical Trial Investigative Site Network Market Size is expected to be worth around US$ 17.2 Billion by 2033, from US$ 8.1 Billion in 2023, growing at a CAGR of 7.8% during the forecast period from 2024 to 2033. North America held a dominant market position, capturing more than a 39.6% share and holds US$ 3.2 Billion market value for the year.

Increasing complexity in clinical trials and the growing demand for faster, more efficient patient recruitment are driving the expansion of the clinical trial investigative site network market. These networks play a vital role in facilitating the recruitment and management of trial participants, ensuring the successful execution of clinical trials across various therapeutic areas.
Rising emphasis on personalized medicine and the need for more diverse patient populations further boost demand for well-established investigative site networks, which can efficiently connect trial sponsors with suitable sites and participants. Opportunities exist in leveraging digital technologies and data analytics to streamline patient recruitment, improve site performance, and enhance real-time monitoring of trial progress.
Recent trends indicate a shift toward decentralized clinical trials, where investigative site networks manage both traditional and virtual trial elements, improving patient access and engagement. In October 2023, Lupus Therapeutics entered into a partnership with AbbVie to support phase III clinical trials of Upadacitinib for systemic lupus erythematosus (SLE).
As part of the collaboration, Lupus Therapeutics will assist with patient recruitment, activating trial sites, and fostering engagement through the Lupus Clinical Investigators Network. This partnership highlights the critical role that investigative site networks play in accelerating clinical trials while optimizing site management and participant retention. As the demand for more efficient and scalable clinical trials grows, the clinical trial investigative site network market is poised for continued growth and innovation.
Key Takeaways
- In 2023, the market for Clinical Trial Investigative Site Network generated a revenue of US$ 8.1 billion, with a CAGR of 7.8%, and is expected to reach US$ 17.2 billion by the year 2033.
- The therapeutic area segment is divided into pain management, oncology, cardiology, and others, with oncology taking the lead in 2023 with a market share of 40%.
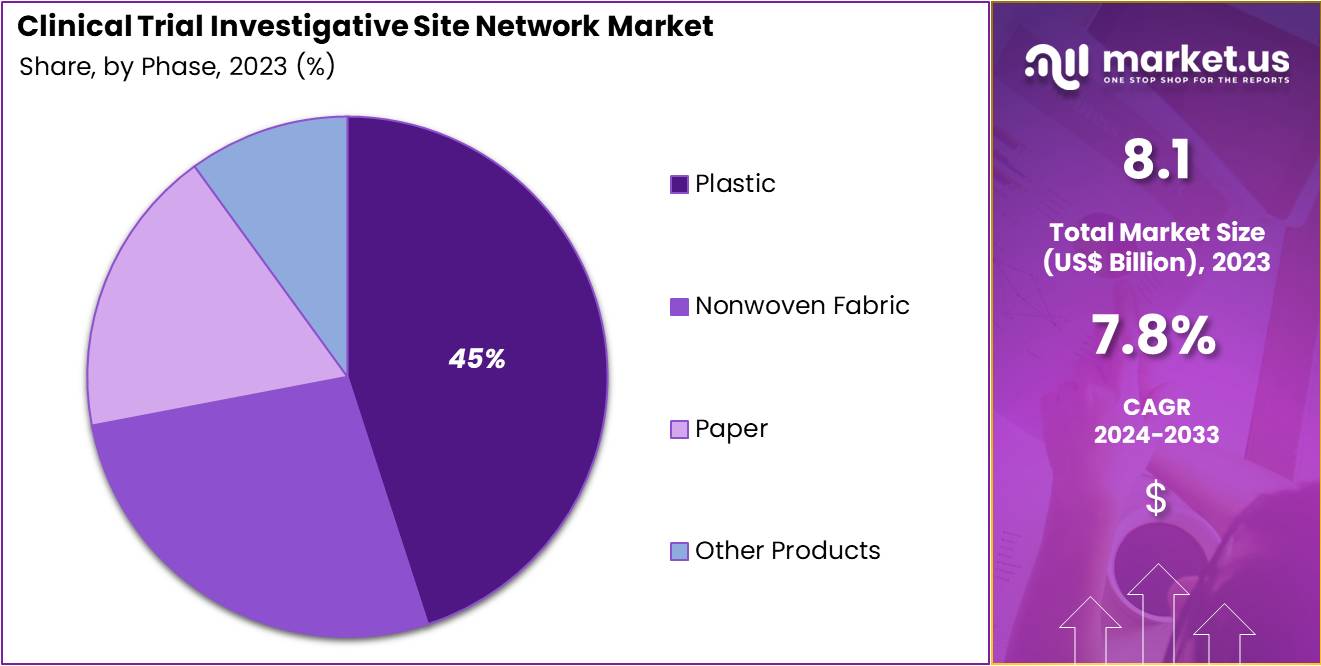
- Considering phase, the market is divided into phase IV, phase III, phase II, and phase I. Among these, phase III held a significant share of 50%.
- Furthermore, concerning the end-use segment, the market is segregated into pharmaceutical & biopharmaceutical companies, medical device companies, and others. The pharmaceutical & biopharmaceutical companies sector stands out as the dominant player, holding the largest revenue share of 60% in the Clinical Trial Investigative Site Network market.
- North America led the market by securing a market share of 39.6% in 2023.
Therapeutic Area Analysis
The oncology segment led in 2023, claiming a market share of 40% owing to the rising global prevalence of cancer and the increasing need for innovative therapies. As cancer treatments become more personalized, clinical trials focused on oncology are likely to expand, requiring more specialized investigative sites. The growing focus on immunotherapy, targeted therapy, and gene therapies has contributed to a surge in clinical trials in oncology.
Additionally, the increasing number of cancer patients, along with advancements in diagnostic techniques, is anticipated to drive more trial participation and investment in oncology-focused trials. These factors, along with a higher demand for precision medicine, will likely fuel the expansion of the oncology segment within the clinical trial site network, offering opportunities for faster patient recruitment and more effective clinical outcomes.
Phase Analysis
The phase III held a significant share of 50% due to the increasing number of drugs and therapies reaching the critical Phase III stage of development. As pharmaceutical and biopharmaceutical companies advance their treatments toward regulatory approval, the demand for robust and efficient clinical trial networks is expected to rise.
Phase III trials, being pivotal in determining the efficacy and safety of treatments, require large-scale patient populations and diverse site locations. The growth of this segment is anticipated to be driven by the need for large-scale, multicenter trials that can recruit participants quickly and gather comprehensive data. As regulatory agencies like the FDA and EMA continue to prioritize faster approval processes, the demand for Phase III trials will increase, strengthening the role of investigative site networks in supporting these essential studies.
End-use Analysis
The pharmaceutical and biopharmaceutical sectors have experienced significant growth, capturing a 60% revenue share due to ongoing advancements in drug discovery and development. These industries are increasingly dependent on clinical trial networks that enhance efficiency through diverse patient inclusion and swift recruitment processes. This dependency is expected to grow as companies broaden their research into new therapeutic domains.
Clinical trials are evolving towards greater complexity, including a focus on personalized medicine. This shift necessitates more specialized investigative sites capable of managing advanced therapies. As research delves deeper into innovative treatments, the requirement for capable trial networks becomes more pronounced.
The expansion of biologics and gene therapies is set to further stimulate demand for sophisticated, adaptable trial networks. These networks are essential for ensuring that new treatments progress efficiently through various trial phases. The development of such networks supports the rapid advancement of new therapeutic options, aligning with the industry’s growth trajectory.
Key Market Segments
By Therapeutic Area
- Pain Management
- Oncology
- Cardiology
- Others
By Phase
- Phase IV
- Phase III
- Phase II
- Phase I
By End-Use
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Others
Drivers
Growing Prevalence of Cancer Driving the Clinical Trial Investigative Site Network Market
The growing prevalence of cancer is significantly driving the clinical trial investigative site network market. According to a January 2024 article by the American Cancer Society, the US is expected to see around 2,001,140 new cancer cases and 611,720 cancer-related deaths. The report also forecasts that the total number of cancer cases will reach 35 million by 2050.
This sharp increase in cancer cases leads to a heightened demand for innovative treatment options and clinical trials. As the need for diverse clinical trial sites to test new therapies rises, the clinical trial investigative site network market is expected to experience robust growth. Investigative site networks play a crucial role in facilitating efficient patient recruitment, managing diverse trial operations, and ensuring timely delivery of results.
The growing cancer patient population will drive pharmaceutical companies and research institutions to expand their clinical trial capacities, making site networks indispensable for meeting the demands of cancer research and treatment development. This trend is likely to contribute significantly to the market’s expansion in the coming years.
Restraints
Regulatory and Ethical Challenges
Regulatory and ethical challenges present a key restraint for the clinical trial investigative site network market. As clinical trials for cancer treatments involve a complex and evolving regulatory environment, it becomes difficult for investigative sites to comply with the stringent guidelines required by regulatory bodies such as the FDA and EMA.
Variations in regulations across regions often result in delays, complicating the process of conducting multi-center or international trials. Moreover, the ethical considerations related to patient consent, especially for vulnerable populations, can slow the approval process and increase costs. Investigative sites must maintain high standards of compliance to protect participants’ rights and safety, which places an additional burden on site operations.
Ethical concerns over patient recruitment practices, data privacy, and adverse event reporting also contribute to the complexities faced by clinical trial networks. These challenges are likely to impede market growth as the need for stricter governance continues to evolve.
Opportunities
Rising Cases of Anxiety and Depression as an Opportunity
Rising cases of anxiety and depression present a significant opportunity for the clinical trial investigative site network market. As mental health disorders continue to increase globally, the demand for clinical trials investigating new treatment options also rises. According to a March 2024 article from the American Psychological Association, around 1 in 20 US adults experience both chronic pain and significant symptoms of anxiety and depression, affecting approximately 12 million Americans or 5% of the adult population.
This growing prevalence of mental health conditions is expected to drive the need for specialized research sites and clinical trials focused on novel therapies for anxiety and depression. The expansion of clinical trial networks dedicated to mental health is likely to accelerate as pharmaceutical companies and researchers work to develop innovative treatments, further fueling market growth.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors have a notable impact on the clinical trial investigative site network market. Economic slowdowns can result in reduced funding for clinical trials, leading to a delay in the launch of new studies and the expansion of trial networks. Additionally, inflationary pressures and fluctuating exchange rates may increase operational costs, affecting profit margins for service providers.
On the other hand, geopolitical instability, such as trade barriers and regulatory changes, may disrupt the flow of essential supplies or hinder international collaborations. Despite these challenges, the growing global focus on advancing healthcare and biotechnology, alongside increasing demand for faster and more efficient trials, continues to drive the market’s expansion. With the rise of personalized medicine and adaptive clinical trials, the network is expected to see significant growth in the coming years.
Trends
Integration of AI Driving the Clinical Trial Investigative Site Network Market
Rising integration of artificial intelligence (AI) is expected to be a key driver of the clinical trial investigative site network market. AI technologies are anticipated to improve the efficiency and accuracy of patient recruitment, data analysis, and trial monitoring. In June 2024, Medidata unveiled Clinical Data Studio, a groundbreaking platform aimed at unlocking the full potential of clinical research data.
This integrated solution offers stakeholders greater control over data quality, facilitating quicker and safer trials for patients. By combining data from both Medidata and external sources, it streamlines decision-making and provides deeper insights into patient data using AI-driven analytics. As a result, clinical trial site networks will become more agile, improving trial success rates and reducing timelines for drug development.
Regional Analysis
North America is leading the Clinical Trial Investigative Site Network Market
North America dominated the market with the highest revenue share of 39.6% owing to regulatory initiatives and technological advancements. The US FDA unveiled new measures in June 2023 aimed at modernizing clinical trials, which is expected to enhance the efficiency and accessibility of these trials. The FDA’s initiative focuses on streamlining regulatory processes and supporting innovative trial models, such as decentralized and adaptive designs, which enable broader patient recruitment and more flexible trial structures.
These changes are expected to foster greater collaboration among clinical trial sites, improve data collection, and reduce trial costs, all of which are crucial for the expansion of the clinical trial site network market. Additionally, the increasing number of clinical trials in areas like oncology, neurology, and rare diseases has contributed to the rise in demand for qualified investigative sites in North America.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
The clinical trial investigative site network market in the Asia Pacific region is anticipated to grow significantly over the forecast period, driven by increasing healthcare investments, rising research activities, and a growing demand for clinical trials in emerging markets. The region is expected to see more collaborative partnerships between international pharmaceutical companies and local clinical sites to accelerate drug development.
As regulatory frameworks evolve, particularly in countries like India, China, and Japan, there will be greater emphasis on decentralized and hybrid trial models, improving access to diverse patient populations. This trend, along with advancements in digital health technologies and clinical trial management software, is likely to expand the clinical trial site network in Asia Pacific, positioning the region as a key hub for global clinical research.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the clinical trial investigative site network market focus on expanding their geographical reach, enhancing operational efficiency, and building strong relationships with pharmaceutical companies to drive growth. Companies invest in streamlining site selection and patient recruitment processes, leveraging technologies such as AI and data analytics to improve trial design and execution.
Strategic collaborations with contract research organizations (CROs) and healthcare institutions help expand their service offerings. Players also emphasize increasing the quality of site management to ensure better regulatory compliance and faster trial timelines. Additionally, expanding into emerging markets allows these companies to tap into untapped patient populations and increase their trial participation.
One key player in the clinical trial investigative site network market is Parexel International Corporation. Parexel offers a range of services, including clinical trial management, regulatory consulting, and patient recruitment, with an extensive network of investigative sites globally.
The company’s growth strategy revolves around enhancing its clinical trial processes through technological innovations, such as AI-driven data analytics, and expanding its reach through strategic partnerships with pharma companies and healthcare providers. By focusing on improving site efficiency and accelerating drug development timelines, Parexel maintains a strong competitive position in the market.
Top Key Players in the Clinical Trial Investigative Site Network Market
- WCG
- SGS North America
- Meridian Clinical Research, LLC
- IQVIA
- George Clinical
- Parexel International Corporation
- Clinical Research Network
- Clara Health
- Alcanza Clinical Research
Recent Developments
- In August 2024: WCG acquired Array, a key partner specializing in content engagement for life sciences organizations. This strategic acquisition is set to strengthen WCG’s service offerings, providing in-depth training materials to help develop a skilled workforce in clinical research.
- In July 2024: SGS North America revealed the expansion of its biologics testing services, increasing its capacity and capabilities to better cater to the needs of the US biopharmaceutical market. This development is aimed at offering enhanced testing solutions to speed up the delivery of biologic products.
Report Scope
Report Features Description Market Value (2023) US$ 8.1 billion Forecast Revenue (2033) US$ 17.2 billion CAGR (2024-2033) 7.8% Base Year for Estimation 2023 Historic Period 2019-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Therapeutic Area (Pain Management, Oncology, Cardiology, and Others), By Phase (Phase IV, Phase III, Phase II, and Phase I), By End-use (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape WCG , SGS North America, Meridian Clinical Research, LLC, IQVIA, George Clinical, Parexel International Corporation, Clinical Research Network, Clara Health, and Alcanza Clinical Research. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Clinical Trial Investigative Site Network MarketPublished date: Dec 2024add_shopping_cartBuy Now get_appDownload Sample
Clinical Trial Investigative Site Network MarketPublished date: Dec 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- WCG
- SGS North America
- Meridian Clinical Research, LLC
- IQVIA
- George Clinical
- Parexel International Corporation
- Clinical Research Network
- Clara Health
- Alcanza Clinical Research









