Antimicrobial Susceptibility Testing Market By Product Type (Automated Laboratory Instruments, Manual Testing Products, and Consumables), By Application (Antibacterial, Anti-parasitic, Antifungal, and Antiviral), By Method (Rapid Automated AST, Disk Diffusion, Gradient Diffusion, Broth Dilution, and Genotypic Methods), By End-user (Hospitals, Pathology/Diagnostic Laboratories, Research & Academic Institutes, and Pharmaceutical & Biotechnology Companies), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Jan 2025
- Report ID: 138381
- Number of Pages: 283
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
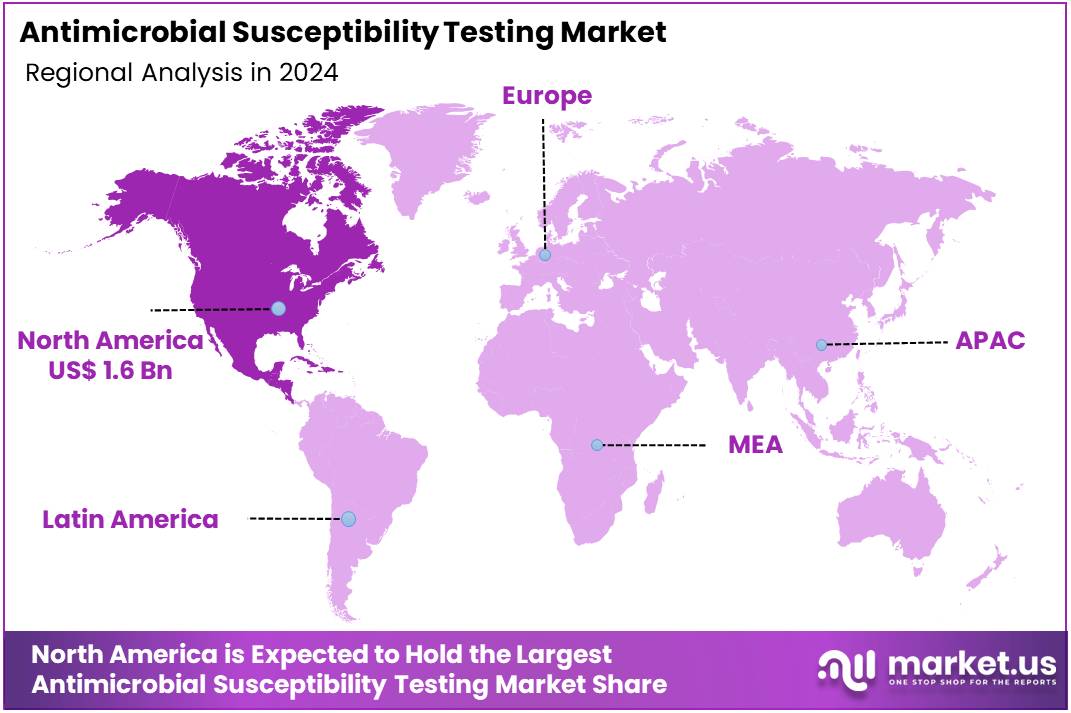
The Antimicrobial Susceptibility Testing Market size is expected to be worth around US$ 7.4 billion by 2034 from US$ 3.9 billion in 2024, growing at a CAGR of 6.6% during the forecast period 2025 to 2034. North America held a dominant market position, capturing more than a 40.2% share and holds US$ 1.6 Billion market value for the year.
Increasing concerns over antibiotic resistance and the growing need for effective treatments are driving the growth of the antimicrobial susceptibility testing (AST) market. AST plays a critical role in identifying the susceptibility of bacteria to various antimicrobial agents, enabling healthcare providers to prescribe the most effective treatments and reduce the risk of resistance development.
The rising incidence of infections caused by multidrug-resistant pathogens underscores the importance of AST in combating global health threats. WHO’s World Antimicrobial Awareness Week (WAAW), held annually from November 18-24, highlights the urgent need for responsible antimicrobial use, further fueling demand for advanced AST methods.
Growing applications of AST span clinical diagnostics, infection control, and research, as it helps in the development of new antibiotics and in monitoring the effectiveness of existing therapies. Recent trends show an increasing shift toward automated and high-throughput AST systems that improve diagnostic efficiency and accuracy while reducing human error.
Additionally, innovations in rapid AST methods, such as molecular diagnostics and microfluidics, present significant opportunities for faster, more cost-effective testing. The adoption of antimicrobial stewardship programs in healthcare settings is also driving market growth, as these programs emphasize the importance of effective infection management and responsible antibiotic use. As antimicrobial resistance continues to rise, the demand for precise, rapid, and efficient AST solutions is expected to keep expanding, creating new growth opportunities in the market.

Key Takeaways
- In 2024, the market for antimicrobial susceptibility testing generated a revenue of US$ 3.9 billion, with a CAGR of 6.6%, and is expected to reach US$ 7.4 billion by the year 2034.
- The product type segment is divided into automated laboratory instruments, manual testing products, and consumables, with consumables taking the lead in 2024 with a market share of 46.7%.
- Considering application, the market is divided into antibacterial, anti-parasitic, antifungal, and antiviral. Among these, antibacterial held a significant share of 50.4%.
- Furthermore, concerning the method segment, the market is segregated into rapid automated AST, disk diffusion, gradient diffusion, broth dilution, and genotypic methods. The disk diffusion sector stands out as the dominant player, holding the largest revenue share of 39.2% in the antimicrobial susceptibility testing market.
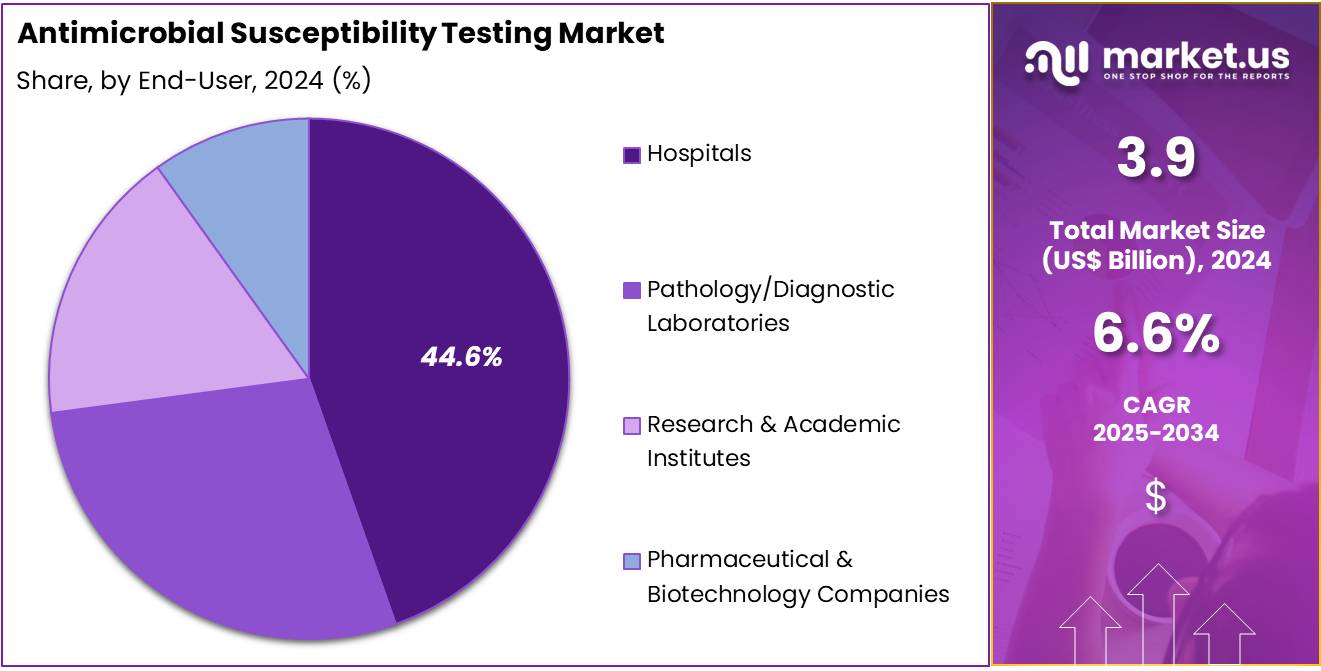
- The end-user segment is segregated into hospitals, pathology/diagnostic laboratories, research & academic institutes, and pharmaceutical & biotechnology companies, with the hospitals segment leading the market, holding a revenue share of 44.6%.
- North America led the market by securing a market share of 40.2% in 2024.
Product Type Analysis
The consumables segment led in 2024, claiming a market share of 46.7% owing to the increasing demand for effective diagnostic tools in identifying antimicrobial resistance. Consumables such as test kits, reagents, culture media, and antimicrobial agents are likely to see rising adoption as healthcare providers and laboratories seek to improve diagnostic accuracy and efficiency.
The growing focus on personalized medicine and the need for rapid, accurate testing methods to guide treatment decisions are expected to drive the demand for consumables. Additionally, the ongoing rise in antimicrobial resistance is likely to increase the need for frequent testing, further contributing to the growth of this segment. As advancements in testing technologies, such as the development of more efficient and cost-effective consumables, continue, this segment is anticipated to expand.
Application Analysis
The antibacterial held a significant share of 50.4% due to the increasing prevalence of bacterial infections and the rise in antibiotic resistance. As healthcare providers focus on the accurate identification of the most effective antibacterial treatments for various infections, the demand for antimicrobial susceptibility testing, particularly for antibacterial agents, is projected to rise.
The growing number of bacterial outbreaks and the need for targeted therapy in hospital settings are likely to contribute to the growth of this segment. Additionally, the rise in chronic conditions that require prolonged use of antibiotics is anticipated to fuel the demand for antibacterial susceptibility testing. Advances in testing methods that improve the speed and accuracy of antibacterial resistance detection are expected to further support the growth of this segment.
Method Analysis
The disk diffusion segment had a tremendous growth rate, with a revenue share of 39.2% owing to its wide adoption in clinical laboratories for assessing bacterial resistance. Disk diffusion is a cost-effective and simple method that remains a staple in antimicrobial susceptibility testing, especially in routine clinical settings.
The increasing emphasis on timely and accurate diagnostics to combat antimicrobial resistance is likely to drive continued use of this method. As hospitals and diagnostic laboratories increasingly adopt standardized protocols for antimicrobial testing, disk diffusion will continue to play a central role in identifying effective treatments for bacterial infections. Furthermore, the development of new antimicrobial agents and the need for effective resistance monitoring are anticipated to sustain the relevance and growth of the disk diffusion segment.
End-user Analysis
The hospitals segment grew at a substantial rate, generating a revenue portion of 44.6% due to the critical role that hospitals play in diagnosing and treating infections, particularly those caused by multidrug-resistant pathogens. Hospitals are expected to remain primary end-users of antimicrobial susceptibility testing services due to the high volume of patients requiring bacterial infection diagnosis and appropriate treatment.
As hospitals expand their infectious disease management programs and enhance their diagnostic capabilities, the demand for antimicrobial susceptibility testing will likely rise. The increasing prevalence of hospital-acquired infections and the growing focus on infection prevention and control strategies are expected to further contribute to the market’s growth in this segment. Additionally, the rising awareness of the importance of precise antibiotic stewardship in hospital settings will likely drive the continued adoption of antimicrobial susceptibility testing in hospitals.
Key Market Segments
By Product Type
- Automated Laboratory Instruments
- Manual Testing Products
- Consumables
By Application
- Antibacterial
- Anti-parasitic
- Antifungal
- Antiviral
By Method
- Rapid Automated AST
- Disk Diffusion
- Gradient Diffusion
- Broth Dilution
- Genotypic Methods
By End-user
- Hospitals
- Pathology/Diagnostic Laboratories
- Research & Academic Institutes
- Pharmaceutical & Biotechnology Companies
Drivers
Rise in Automated Testing Driving the Antimicrobial Susceptibility Testing Market
Rising adoption of automated testing is anticipated to drive the antimicrobial susceptibility testing market significantly. In April 2022, COPAN Diagnostics showcased a full suite of automated solutions for identification and susceptibility testing at ECCMID 2022. Automated systems streamline workflows, reducing manual errors and improving the precision of results.
These technologies enable faster identification of antimicrobial resistance, which is critical for timely and effective patient care. Laboratories increasingly adopt automated platforms to manage high sample volumes efficiently, addressing growing diagnostic demands. Integration of artificial intelligence and machine learning enhances the predictive capabilities of automated testing systems.
Expanding applications of automation in point-of-care diagnostics improve accessibility and speed of resistance detection. Pharmaceutical companies leverage these advancements to support drug development and monitor the effectiveness of new antibiotics. Automated systems also enhance data management and reporting, facilitating better decision-making in clinical settings. Collaboration between diagnostic companies and healthcare providers accelerates the implementation of advanced automated solutions. These trends underscore the transformative impact of automation in advancing antimicrobial resistance detection.
Restraints
High Costs Are Restraining the Antimicrobial Susceptibility Testing Market
High costs associated with antimicrobial susceptibility testing are restraining the market. Advanced automated systems and consumables require significant investment, limiting adoption in smaller laboratories with constrained budgets. The expense of maintaining and calibrating these systems further adds to operational costs. In resource-limited settings, affordability remains a critical challenge, reducing access to state-of-the-art testing solutions.
Limited availability of skilled personnel to operate complex systems increases training expenses for laboratories. Variability in reimbursement policies across regions discourages widespread adoption of advanced susceptibility testing. The high cost of consumables, including reagents and cartridges, increases the per-test price, creating financial constraints for healthcare facilities. Addressing these issues requires cost-efficient innovations and targeted government subsidies to improve accessibility and affordability of testing solutions.
Opportunities
Increasing R&D Activities as an Opportunity for the Antimicrobial Susceptibility Testing Market
Increasing R&D activities are anticipated to create significant opportunities for the antimicrobial susceptibility testing market. A December 2021 study published by NCBI demonstrated that direct rapid antibiotic susceptibility tests could detect resistance within six hours of a Gram smear result. Such advancements accelerate the selection of appropriate antibiotics for conditions like bacteremia in COVID-19 patients.
Pharmaceutical companies prioritize research to develop rapid and accurate diagnostic solutions to combat rising antimicrobial resistance. Integration of novel technologies, such as microfluidics and next-generation sequencing, enhances the precision and scalability of susceptibility testing. Collaborative efforts between research institutions and industry leaders accelerate the commercialization of innovative testing methods.
Expanding government funding and public-private partnerships support research aimed at improving diagnostic tools for infectious diseases. Development of portable and point-of-care testing devices expands the market’s reach in remote and underserved regions. These trends highlight the pivotal role of R&D in driving innovation and improving the efficiency of antimicrobial resistance detection.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors significantly impact the antimicrobial susceptibility testing market. On the positive side, the increasing global burden of antimicrobial resistance (AMR) and the rising awareness of its implications drive demand for effective susceptibility testing methods. Governments and healthcare organizations are investing more in advanced diagnostic technologies to combat AMR, promoting market growth. However, economic downturns can lead to reduced healthcare spending, which may delay the adoption of new testing technologies.
Geopolitical factors, such as trade barriers, regulatory discrepancies, and political instability, can disrupt the supply chain for testing products and reagents. Moreover, varying regulatory standards across regions may create challenges for manufacturers seeking to enter new markets. Despite these obstacles, the growing focus on AMR and the ongoing improvements in diagnostic capabilities ensure a continued upward trajectory for the market.
Trends
Surge in Approval of Novel Testing Systems Driving the Antimicrobial Susceptibility Testing Market
The Antimicrobial Susceptibility Testing (AST) market is experiencing substantial growth driven by increasing approvals of novel testing systems. These systems are designed to meet the high demand for rapid and accurate diagnostics, essential for effective treatment decisions. The rise in antimicrobial resistance underscores the need for these advanced diagnostic tools, which promise quicker results and enhanced reliability.
In August 2022, a significant development occurred when bioMérieux received FDA approval for its SPECIFIC REVEAL Rapid Antimicrobial Susceptibility Test System. This approval marks a pivotal step in the adoption of rapid AST methods. Such advancements are expected to boost the usage of rapid testing technologies, particularly in the management of antimicrobial resistance diseases.
The market is poised for further expansion as more innovative testing systems gain regulatory approval. This trend is expected to improve the speed and effectiveness of diagnostics for antimicrobial resistance (AMR). As healthcare providers increasingly rely on these advanced systems, the overall capacity to manage and treat AMR effectively is anticipated to enhance significantly.
Regional Analysis
North America is leading the Antimicrobial Susceptibility Testing Market
North America dominated the market with the highest revenue share of 40.2% owing to the increasing prevalence of antimicrobial resistance (AMR), rising demand for rapid diagnostic tools, and advancements in testing technologies. As antibiotic-resistant infections become more prevalent, the need for accurate and timely susceptibility testing has grown to ensure effective treatment options are available.
In January 2023, NanoSynex Ltd. announced its plan to commercialize its innovative rapid AST, a development that is poised to streamline the testing process and reduce turnaround times. The growing focus on reducing healthcare-associated infections, improving patient outcomes, and supporting stewardship programs has also driven the demand for advanced AST solutions.
Additionally, increased awareness about the dangers of antimicrobial resistance, along with rising investments in healthcare infrastructure and diagnostics, has further fueled market growth in North America. The region’s strong healthcare ecosystem, which includes a high rate of adoption of new technologies, positions the antimicrobial susceptibility testing market for continued expansion.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to grow with the fastest CAGR owing to rising healthcare concerns, increasing antibiotic resistance, and improvements in diagnostic capabilities. The growing incidence of bacterial infections, coupled with the escalating threat of antimicrobial resistance in countries like China, India, and Japan, is likely to push the demand for effective AST solutions.
In July 2024, Shionogi launched the in vitro diagnostic product “Shionogi MIC Dry Plate Cefiderocol” to assess sensitivity to treatment for gram-negative bacterial infections, a critical advancement in drug-sensitivity testing. This innovation is anticipated to enhance the appropriate utilization of Fetroja, a drug used for multidrug-resistant bacterial infections, and contribute to the growth of the antimicrobial susceptibility testing market in Asia Pacific.
As healthcare systems across the region continue to evolve, with increasing emphasis on improving infection control and diagnostic precision, the market for AST solutions is projected to experience robust growth. The expanding focus on improving antimicrobial stewardship and regulatory support for new diagnostic tools will likely further fuel market expansion.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the antimicrobial susceptibility testing market focus on developing automated systems and advanced assays to improve accuracy and efficiency in detecting microbial resistance. Companies invest in R&D to create rapid and high-throughput diagnostic solutions, catering to the needs of hospitals and clinical laboratories.
Collaborations with pharmaceutical firms and research organizations help expand the scope of testing applications, including novel antibiotic development. Geographic expansion into regions with rising antimicrobial resistance challenges supports market penetration. Many players also emphasize regulatory compliance and affordability to ensure accessibility and trust among healthcare providers.
BioMérieux SA is a leading company in this market, offering innovative solutions such as the VITEK® series for rapid antimicrobial susceptibility testing. The company combines advanced technology with user-friendly platforms to deliver precise and reliable diagnostics. BioMérieux’s global presence and strong focus on combating antimicrobial resistance solidify its position as a key player in the diagnostics industry.
Top Key Players in the Antimicrobial Susceptibility Testing Market
- Qualigen Therapeutics
- Hi-Media Laboratories Pvt. Ltd.
- ELITechGroup
- Danaher Corporation
- Creative Diagnostics
- bioMérieux SA
- BD
Recent Developments
- In February 2023, bioMérieux S.A. launched MAESTRIA, a new-generation microbiology software designed to streamline various workflow steps, including the input of antimicrobial susceptibility testing (AST) results. This software aims to reinforce bioMérieux’s position in infectious disease diagnostics by integrating cutting-edge technology for efficient data management in AST processes.
- In May 2022, Qualigen Therapeutics confirmed that NanoSynex Ltd, a company in which they had just secured a majority stake, was highlighted at BioMed Israel. The focus of NanoSynex’s innovative technology is on expediting the time for antimicrobial susceptibility test results, aiming to reduce it by up to six times.
Report Scope
Report Features Description Market Value (2024) US$ 3.9 billion Forecast Revenue (2034) US$ 7.4 billion CAGR (2025-2034) 6.6% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Automated Laboratory Instruments, Manual Testing Products, and Consumables), By Application (Antibacterial, Anti-parasitic, Antifungal, and Antiviral), By Method (Rapid Automated AST, Disk Diffusion, Gradient Diffusion, Broth Dilution, and Genotypic Methods), By End-user (Hospitals, Pathology/Diagnostic Laboratories, Research & Academic Institutes, and Pharmaceutical & Biotechnology Companies) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Qualigen Therapeutics, Hi-Media Laboratories Pvt. Ltd., ELITechGroup, Danaher Corporation, Creative Diagnostics, bioMérieux SA, and BD. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Antimicrobial Susceptibility Testing MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample
Antimicrobial Susceptibility Testing MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Qualigen Therapeutics
- Hi-Media Laboratories Pvt. Ltd.
- ELITechGroup
- Danaher Corporation
- Creative Diagnostics
- bioMérieux SA
- BD









