Global AI in Clinical Trials Market By Offering (Software and Services), By Technology (Machine learning, Deep learning and Supervised), By Application (Cardiovascular, Metabolic, Oncology and Infectious diseases), By End User (Pharmaceutical companies, Biotechnology companies, and Contract Research Organizations (CROs)), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Aug 2025
- Report ID: 116297
- Number of Pages: 352
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
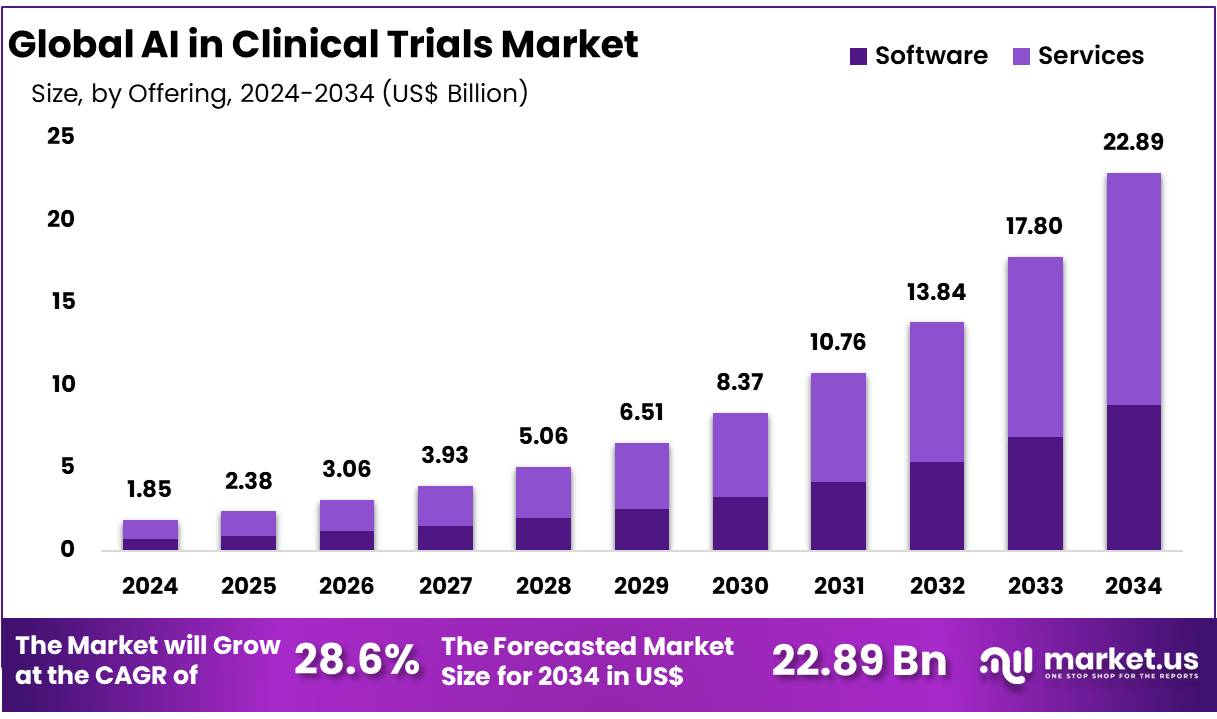
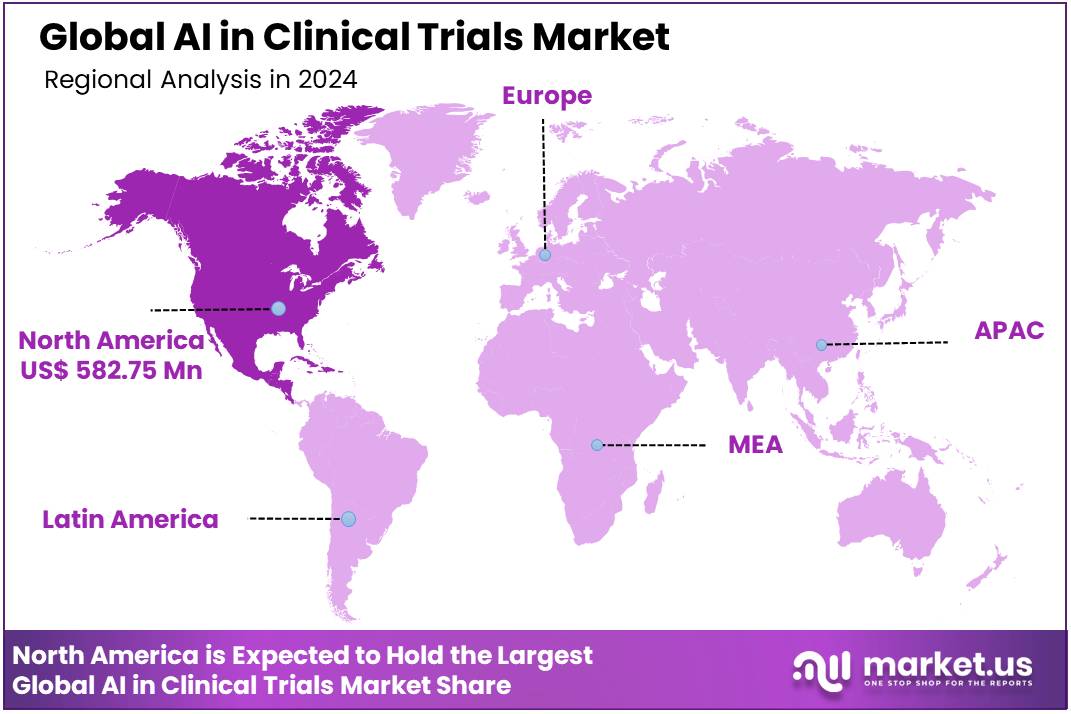
Global AI in Clinical Trials Market size is expected to be worth around US$ 22.89 Billion by 2034 from US$ 1.85 Billion in 2024, growing at a CAGR of 28.6% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 31.5% share with a revenue of US$ 582.75 Billion.
AI in Clinical Trial market encompasses creation, application, and commercialization of AI technologies, togetherly aimed at enhancing the efficiency, precision and efficacy of clinical research. Being cost and time intensive, clinical trial account for nearly 50% of the time and expense during the process of drug development. Many a times, clinical trials encounter failure due to inadequate study design, improper subject stratification, high participant attrition and incomplete patient recruitements. Thus, the major market players in pharmaceutical industries are exploring solutions to avoid failures and streamline clinical trial stages through incorporation of AI solutions.
AI based drug development is one such innovative solution, revolutionizing traditional methods, especially in clinical trials. Large volumes of data can be integrated and analyzed aided by artificial intelligence, allowing the trial sponsors to optimize future research activities. Thus, the ability of AI to enhance and refine the entire clinical drug development process drives the market for AI in clinical trials to a large extent. In addition, the growing investments of investors in AI based clinical trials further thrust the market growth during the projection period.
In January 2024, Accenture made a strategic investment in QuantHealth via Accenture Ventures. QuantHealth, an AI-driven clinical trial design company, utilizes advanced technology to accurately predict trial outcomes and reduce the potential costs associated with drug discovery.
Key Takeaways
- Market Size: AI In Clinical Trial Market was valued at the USD 1.85 Billion in 2024 and is projected to register a substantial growth of the USD 22.89 Billion by 2034.
- By Offering Analysis: A largest market share of 61.3% is held by services segment.
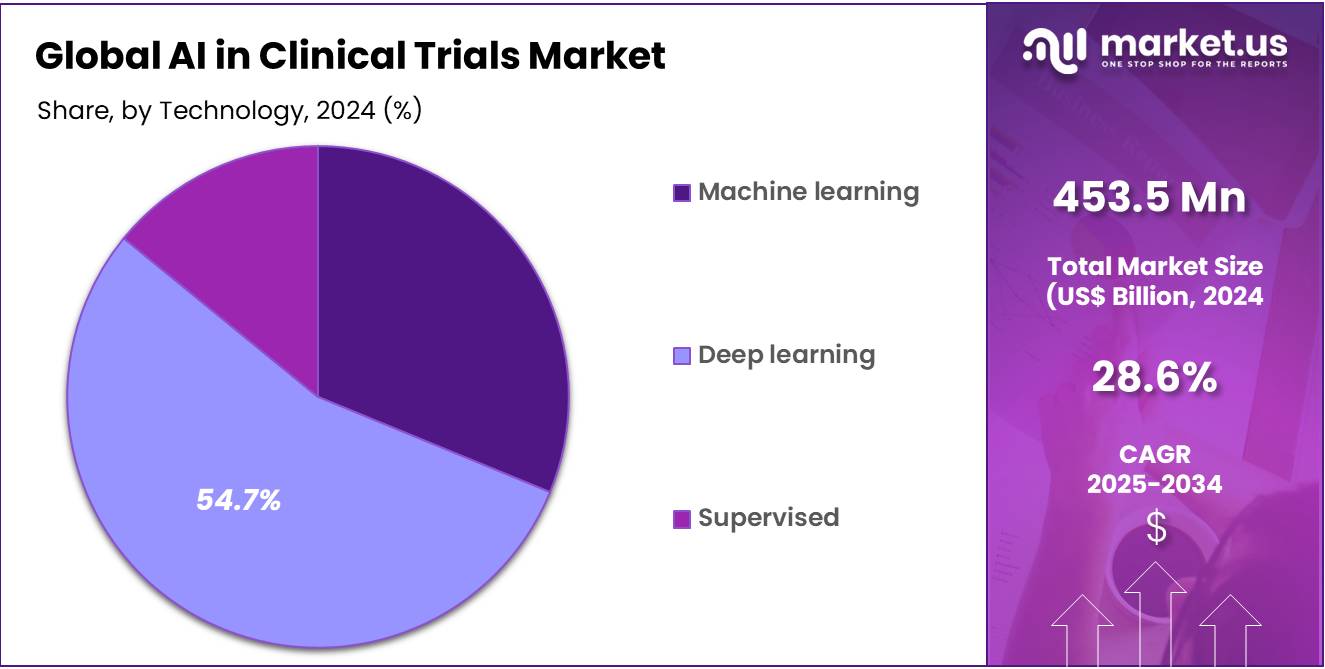
- Technology Analysis: Deep learning segment accounts 54.7% market share in 2024.
- Application Analysis: The oncology segment to dominate 45.9% market share.
- End User analysis: pharmaceutical companies, allow them to procure a measurable market share of 65.8% in the year 2024.
- Regional Analysis: North america region dominate 31.5% market share in 2024.
Offering Analysis
Based on offerings, the market is broadly categorized into software and sevices segment. A largest market share of 61.3% is held by services segment, dominating the AI in clinical trial market in the year 2024. Distinctive requirement and goals of various clinical trials and healthcare institutions are met by virtue of service segment providing customized solutions. The segment provides satisfactory outcomes to the stakeholders by negotiating regulatory environment around clinical trials, guaranting that AI applications are in accordance with relevant healthcare laws and business norms.
In May 2025, ClinTrial Research (CTR), a prominent site management organization (SMO), announced a strategic partnership with Trially®, a cutting-edge HIPAA-compliant AI matching technology. After six months of collaboration, the partnership has led to notable improvements in patient identification, enrollment speed, and operational efficiency.
Technology Analysis
Depending upon the technology analysis, the market is fragmented into machine learning, deep learning and supervised segments. A market share of 54.7% is held by deep learning segment, overshadowing the AI in clinical trials market in the year 2024. The dominance of deep learning segment gets stronger with the rising ultimatum for innovatory data analytics and growing personalized healthcare. Being a subset of machine learning, deep learning allow processing and interpreting convulated datasets, making it appropriate for complexities of clinical trial data.
In addition, the segment enables more accurate diagnostics, patient compartmentalization and predictive modelling, by analyzing abundant amount of data such as medical images, genomic data, and patient records. AI powered clinical trial not only assists drug discovery but is also used for testing the discovered molecules in pre-clinical animals or invitro models, i.e., AI is used in around 50-62% of the entire value chain of drug development.
For instance, in May 2025, Rein Therapeutics, a biopharmaceutical company focused on developing first-in-class medicines for orphan pulmonary and fibrosis conditions, has announced a collaboration with Qureight Ltd, a Core Imaging Laboratory specializing in deep-learning image analytics. The partnership will integrate Qureight’s deep-learning platform into Rein’s upcoming Phase 2 trial of LTI-03, a novel, multi-pathway, Caveolin-1-related peptide, for the treatment of idiopathic pulmonary fibrosis (IPF).
Application Analysis
The AI in clinical trial market is bifurcated into Cardiovascular, metabolic, Oncology and Infectious diseases segments, based on its applications. Artificial intelligence being highly adopted to diagnose and treat several types of cancers, the oncology segment dominated the AI in clinical market, capturing a prominent market share of 45.9% in the year 2024. The dominance of the segment is highly attributed to rising prevalence of cancer globally coupled with hefty number of drug trials conducted in the oncology field.
In addition, the major industrial players are engrossed in development and adoption of oncology based AI tools for clinical trials. To exemplify, a collaboration was held between Deep Lens and Hematology-Oncology Associates of Central New York with the aim to expand drug trial program. VIPER by Deep Lens assisted to identify qualified patients by pre-screenig them for the purpose of clinical trials.
In April 2025, Onc.AI, a digital health company developing advanced AI-driven clinical management solutions for oncology, has announced that findings from its recent collaboration with global biopharma company GSK will be presented at the 2025 American Association for Cancer Research (AACR) Annual Meeting. The study, which externally evaluated Onc.AI’s FDA breakthrough-designated Serial CTRS AI model, was conducted in GSK’s GARNET Phase I clinical trial (NCT02715284) Cohort E, enrolling patients with advanced non-small cell lung cancer (NSCLC) treated with dostarlimab, GSK’s anti-PD-1 checkpoint inhibitor.
End User Analysis
Based on end user, the market is fragmented into Pharmaceutical companies, Biotechnology companies and Contract Research Organizations (CROs) segments. The highest use of AI algorithms by pharmaceutical companies, allow them to procure a measurable market share of 65.8% in the year 2024.
AI significantly assist the pharmaceutical companies to scrutinize large amount of data, thereby accelerating medication development process enabling pattern recognition and optimization of clinical trials. In addition, there is high adoption of AI by pharmaceutical companies as it allow to detect and assuage possible fortuity associating the clinical studies.
Key Market Segments
By Offering
- Software
- Services
By Technology
- Machine learning
- Deep learning
- Supervised
By Application
- Cardiovascular
- Metabolic
- Oncology
- Infectious diseases
By End User
- Pharmaceutical companies
- Biotechnology companies
- Contract Research Organizations (CROs)
Drivers
Rising demand of AI tools in pharmaceutical firms and rising investments
The AI tools plays a pivotal role in ameliorating the efficacy and accuracy of testing, optimizing clinical trial outcomes and and acceleration of drug development in pharmaceutical companies. There comes more accuracy in clinical data analysis, ease of trial design, enhancement in patient enrollment and retention, cost and time reduction, and personalized medicines aided by AI software in clinical trial.
Stakeholders have a keen interest in pharmaceutical firms by virtue of the ability of AI to automate and streamline laborious tasks, identify patterns and trends in complex datasets and improve decision making process, rising the ultimatum of AI solutions in pharma companies. In addition, the rising investment from major market players is also helping the market to grow on an amazing scale. An investment of the USD 23.9 million was received by Antidote Technologies to expand its digital patient engagement programs coupled with clinical trial recruitement services.
Restraints
High cost can be constraining
The high cost of adopting AI in clinical trial pocedures can be prohibitive for many small scale firms. This is mainly due to enoumous cost required for improving infrastructure, acquisition of technology and development of software. The specialized AI tools needed for data analysis, predictive modeling in clinical trials and patient recruitment are expensive to develop and implement. The cost is escalates further as these advance AI applications frequently need cooperation from data scientists, healthcare providers and technology specialists.
Thus, this significantly constrains the adoption of AI tools in clinical trial market. AI applications in clinical trials has to comply with stringent regulations in order to know AI systems are appropriately adhering to the criteria provided by regulatory authorities. Thus, this causes delay in the creation and application of AI solutions for clinical trials, thereby limiting its use in the market.
Opportunities
Govenrment initiatives creates lucrative opportunities for market growth
There is an escalated demand for AI based technologies, tools and solutions due to heightened government support and stringent regulations related to clinical trials. In addition, there is rising recognition of clinical trials by administrations of numerous emerging and emerged nations for encouraging patient participation. This creates tremendous opportunities for market players to flourish and create anundant revenues during the projection period.
For instance, with the aim to accelerate development of new drug candidates for hospitalized COVID-19 patients, the UK government a clinical trial program, “ACCORD”-The Accelerating COVID 19 Research and Development Platform. In June 2025, the U.S. Food and Drug Administration (FDA) launched Elsa, a generative artificial intelligence (AI) tool aimed at enhancing the efficiency of its employees, from scientific reviewers to investigators. This innovative tool modernizes the agency’s functions, harnessing AI capabilities to improve its services for the American public.
Impact of Macroeconomic / Geopolitical Factors
The demand for AI solutions is significantly influenced by various important factors such as increasing disease prevalence, healthcare priorities, and demographic shifts, leading to an escalating demand for innovative technologies, aiming to enhance effectiveness of clinical research.
The accessibility to heathcare data and expertise obligatory for AI execution can be severely affected by the economic incongruity across the countries. Investors can decline their investments due to economic downturns leading to reduced fundings for startups and research projects within the healthcare settings. Thus, these facets leads to slowing down the development and adoption of AI technologies.
Latest Trends
AI-Driven Decentralized Clinical Trials
One of the most significant trends in the AI-driven clinical trial market is the rise of decentralized clinical trials (DCTs). Traditional clinical trials often require patients to visit physical sites for assessments, which can be inconvenient and time-consuming. With advancements in AI, clinical trials are increasingly moving toward a decentralized model, allowing participants to take part remotely. AI-powered tools can enable remote monitoring, data collection, and virtual assessments, reducing the need for patients to travel to trial sites.
This trend has been accelerated by the COVID-19 pandemic, which demonstrated the feasibility and advantages of conducting trials remotely. AI is used to analyze data from wearable devices, mobile apps, and remote monitoring tools to track patient progress, ensuring continuous real-time monitoring.
Additionally, AI algorithms help analyze and interpret large volumes of data generated by these decentralized systems, providing valuable insights without the need for traditional on-site visits. DCTs offer several benefits, including improved patient recruitment, access to a more diverse participant pool, and reduced costs and time. This trend is expected to continue gaining momentum, making clinical trials more accessible, efficient, and patient-centric.
Regional Analysis
North America is leading the AI in Clinical Trials Market
Being a home to numerous AI technology providers as well as leading start up firms, North America overshadowed the global AI in clinical trial market capturing a remarkable market share of 31.5% in the year 2024. The regions’ prominence is reflective of the well established pharmaceutical industries such as Abbott Laboratories, Johnson and Johnson and Pfizer, escalating research and development spendings, large pool of clinical trial service providers and enhanced investments in biosimilars and biologics area in the region.
For instance, bfLEAP, a proprietary AI platform was developed by a U.S based startup, ‘Bullfrog AI’, enabling precision medicine. Furthermore, a fastest growth rate of 31.5% is anticipated to register by Asia-Pacific, owing to increasing penetration of AI based tools combined with suitable government initiatives with the aim to adopt AI in diverse healthcare fields. In addition, Asia is witnessing elevating enrolments for clinical trials in comparison with Europe and North America, owing to the existence of huge patient pool and low trial cost.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the AI in Clinical Trials market includes Phesi, Intelligencia, Deep Lens, Halo Health Systems, Pharmaseal, Koneksa Health, BioSymetrics, Google-Verify, IBM Watson, Symphony AI, BioAge Labs, Inc, Ardigen, Medidata, IQVIA, Unlearn.AI, PathAI, ConcertAI, Norstella, BenevolentAI, Insilico Medicine, Exscientia, XtalPi, Opyl, Deep Lens, Clinithink, and Other key players.
Phesi leverages its AI-powered Trial Accelerator™ platform, which integrates data from over 132 million patients to simulate clinical trials and optimize decision-making. This platform aids in protocol design, investigator site selection, and patient recruitment, aiming to reduce trial costs and improve success rates. Intelligencia AI specializes in enhancing clinical development through AI-driven solutions that assess the probability of success (PoS) for drugs across various therapeutic areas.
Their transparent, data-driven models support decision-making by identifying underlying risks and predicting outcomes, thereby de-risking drug development processes. Deep Lens offers VIPER, an AI-based platform that automates patient matching for clinical trials by analyzing electronic medical records, genomic data, and pathology reports in real time. This approach accelerates recruitment, reduces study timelines, and enhances the efficiency of oncology clinical trials.
Top Key Players
- Phesi
- Intelligencia
- DEEP LENS AI
- Halo Health Systems
- Pharmaseal
- Koneksa Health
- BioSymetrics
- Google-Verify
- IBM Watson
- Symphony AI
- BioAge Labs, Inc
- Ardigen
- Medidata
- IQVIA
- AI
- PathAI
- ConcertAI
- Norstella
- BenevolentAI
- Insilico Medicine
- Exscientia
- XtalPi
- Opyl
- Deep Lens
- Clinithink
- Other key players
Recent Developments
- In April 2025, Worldwide Clinical Trials, a global contract research organization (CRO), has introduced an advanced artificial intelligence (AI) tool to improve clinical trial optimization. This innovative service segments patient populations to identify those most likely to contribute to successful trial outcomes, resulting in fewer patients needed per trial, shortened timelines, reduced costs, and higher success rates for sponsors.
- In August 2023, Texas Tech University Health Sciences Center (TTUHSC) and Deep 6 AI have partnered to integrate artificial intelligence (AI) into TTUHSC’s electronic medical record (EMR) system. This collaboration aims to enhance patient access to clinical trials by leveraging AI to identify eligible patients more efficiently.
- In July 2023, Phase II clinical trial was initiated by Insilico Medicine to evaluate first fully AI designed drug, INSO18_055, for the treatment of idiopathic pulmonary fibrosis.
Report Scope
Report Features Description Market Value (2024) US$ 1.85 Billion Forecast Revenue (2034) US$ 22.89 Billion CAGR (2025-2034) 28.6% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Offering (Software and Services), By Technology (Machine learning, Deep learning and Supervised), By Application (Cardiovascular, Metabolic, Oncology and Infectious diseases), By End User (Pharmaceutical companies, Biotechnology companies, and Contract Research Organizations (CROs)) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Phesi, Intelligencia, DEEP LENS AI, Halo Health Systems, Pharmaseal, Koneksa Health, BioSymetrics, Google-Verify, IBM Watson, Symphony AI, BioAge Labs, Inc, Ardigen, Medidata, IQVIA, Unlearn.AI, PathAI, ConcertAI, Norstella, BenevolentAI, Insilico Medicine, Exscientia, XtalPi, Opyl, Deep Lens, Clinithink, and Other key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  AI In Clinical Trial MarketPublished date: Aug 2025add_shopping_cartBuy Now get_appDownload Sample
AI In Clinical Trial MarketPublished date: Aug 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Phesi
- Intelligencia
- DEEP LENS AI
- Halo Health Systems
- Pharmaseal
- Koneksa Health
- BioSymetrics
- Google-Verify
- IBM Watson
- Symphony AI
- BioAge Labs, Inc
- Ardigen
- Medidata
- IQVIA
- AI
- PathAI
- ConcertAI
- Norstella
- BenevolentAI
- Insilico Medicine
- Exscientia
- XtalPi
- Opyl
- Deep Lens
- Clinithink
- Other key players









