Global ADME Toxicology Testing Market By Technology (Cell Culture, High Throughput Screening, Molecular Imaging, OMICS Technology), By Application (Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity), By Product (Instruments, Software Solutions, Assays Systems, Reagents), By Method (In-Vivo, In-Vitro, In-Silica,) By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2023-2032
- Published date: July 2024
- Report ID: 55225
- Number of Pages: 357
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
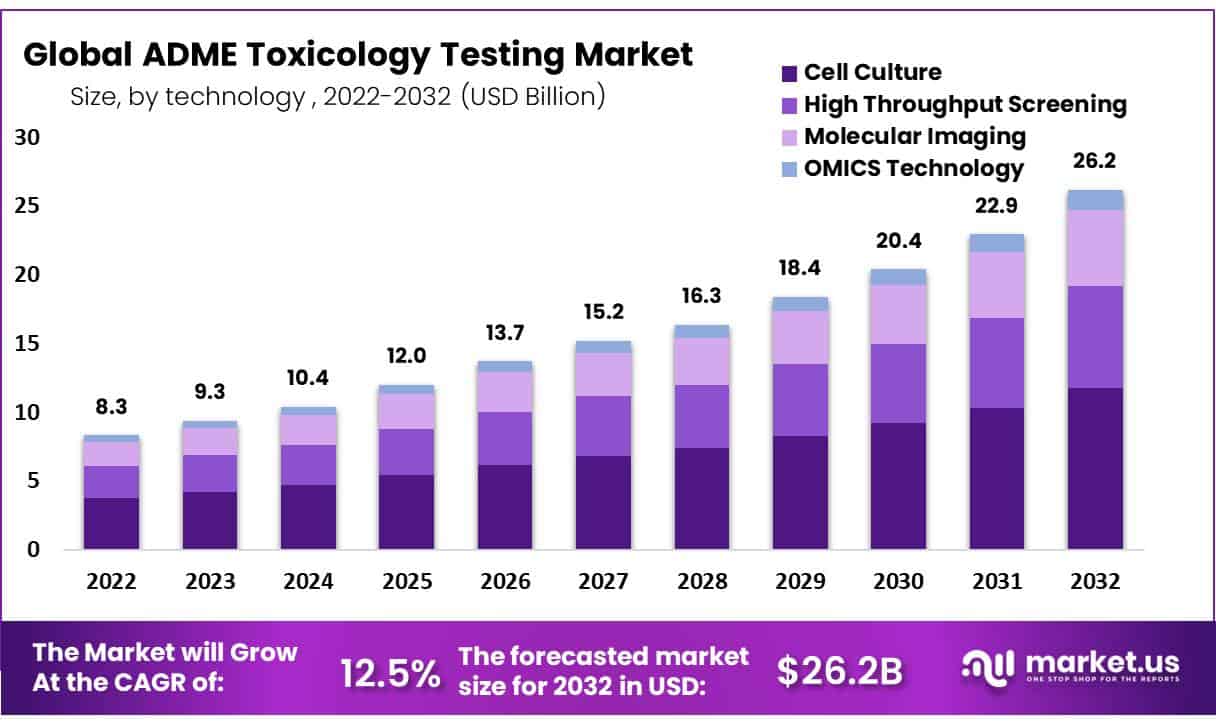
The Global ADME Toxicology Testing Market size is expected to be worth around USD 26.2 Billion by 2032 from USD 9.3 Billion in 2023, growing at a CAGR of 12.5% during the forecast period from 2023 to 2032.
The Global ADME Toxicology Testing Market studies drug and chemical Absorption, Distribution, Metabolism, and Excretion (ADME) to assess their toxicity. This testing is essential in the drug discovery and development process as it helps identify potential risks and guarantees product safety.
The market for ADME toxicology testing is being driven by factors such as an increasing number of drug development programs, the need for accurate and dependable toxicity testing methods, and personalized medicine’s rising popularity. Furthermore, chronic diseases and an aging population are expected to fuel the demand for new drugs and accelerate market expansion.
The demand for innovative medications and biological products increases as disease prevalence increases. This is one of the primary reasons why ADME toxicology testing is becoming more widely utilized, to avoid candidate medications from failing late-stage clinical trials. Researchers use it to assess the potential of medications before seeking regulatory approval.
The Food and Drug Administration (FDA) recently issued guidance documents to provide instructions regarding ADME features when assessing drug candidates, an important development in this industry. As an alternative to traditional in vitro assays and in vivo investigations, software that can automatically compute ADME is becoming more widely used.
ADME toxicology testing is being utilized to assess the efficacy of existing medications as an alternative treatment, which is fueling market growth in light of COVID-19’s widespread outbreak and lack of a viable vaccine or treatment.
Key Takeaways
- Market Size: Global ADME Toxicology Testing Market size is expected to be worth around USD 26.2 Billion by 2032 from USD 9.3 Billion in 2023.
- Market Growth: The market growing at a CAGR of 12.5% during the forecast period from 2023 to 2032.
- Technology Analysis: Cell culture technology was estimated to be the dominant segment, with 45% of the market share.
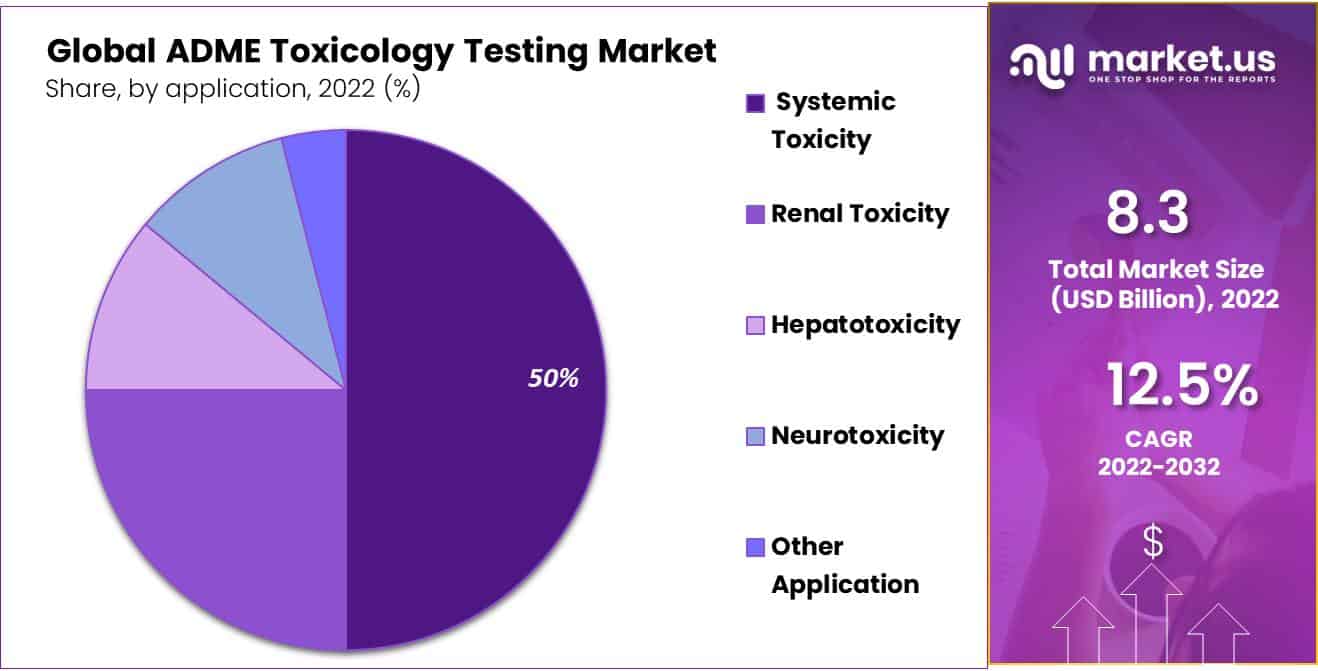
- Application Analysis: Systemic toxicity dominates the market with 50% of the market share i 2022.
- Method Analysis: The Cellular Assay dominates the market with 45% of the market share.
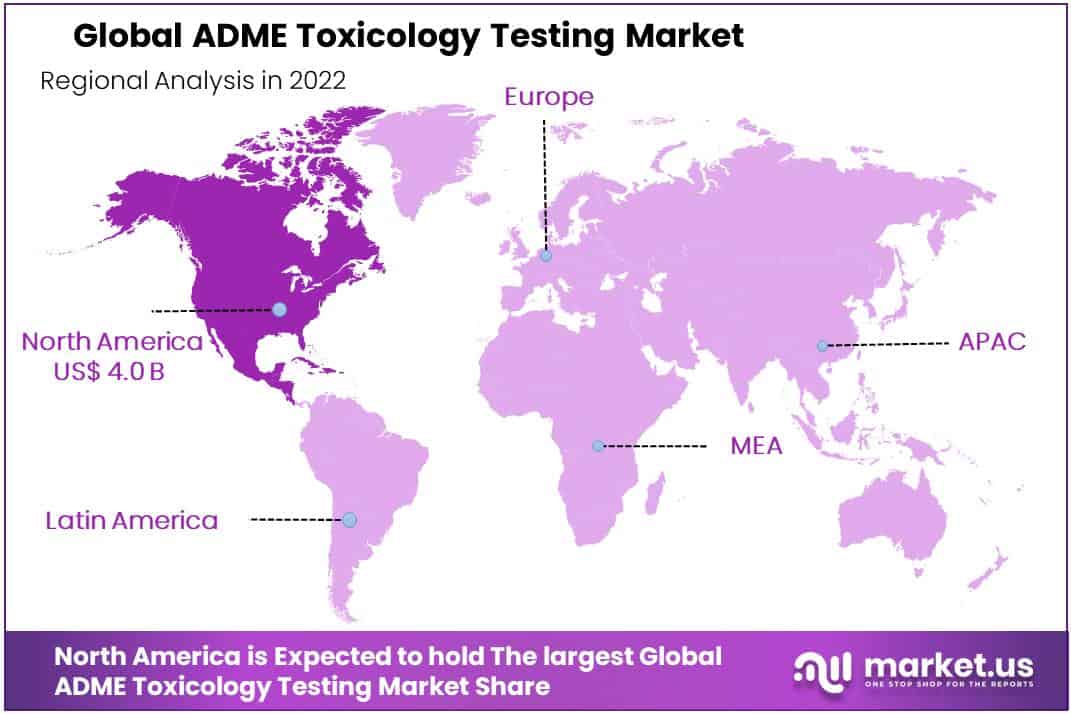
- Regional Analysis: The largest share in the market will be dominated by North America, with 49% of the market share.
- Regulatory Influence: Stringent regulations by health authorities like the FDA and EMA are mandating comprehensive ADME toxicology testing, thereby boosting market demand.
- Pharmaceutical Industry Demand: The pharmaceutical and biotechnology sectors are the primary drivers of this market, as they invest heavily in ADME toxicology testing to ensure drug safety and efficacy.
- Future Prospects: The market is anticipated to grow further with the rising adoption of automated and AI-driven ADME toxicology testing solutions, which enhance predictive accuracy and reduce time-to-market for new drugs.
By Technology Analysis
The Cell Culture Segment is Dominant in the ADME Toxicology Testing Market.
Based on technology, the market for ADME toxicology testing is segmented into Cell Culture, High Throughput Screening, Molecular Imaging, and OMICS Technology. Cell culture technology was estimated to be the dominant segment, with 45% of the market share. Due to their capacity for producing visual data and testing with minimal amounts, fluorometric imaging plate reader assays have become the go-to cell-based assays. Ultra ADME toxicology testing, the latest innovation in this area, allows businesses to produce more work in less time while transitioning from 2D cell cultures to 3D ones.
Three-dimensional cells can be made more human-like by studying their environment and other modifications, leading to drug design and discovery. To fully automate testing, hardware and software must be developed that allows this and will also contribute to future market growth. Moreover, chemical libraries need to be expanded in order to enable proactive identification and testing of chemical identity hits.
By Application Analysis
The Systemic toxicity application segment is Dominant in the ADME Toxicology Testing Market.
By application, the market is further divided into Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, and Other Applications. Systemic toxicity dominates the market with 50% of the market share. This results from the molecule’s growing propensity for systemic circulation in the context of medication delivery. When a substance, especially a neurotoxin or a neurotoxicant, alters the nervous system’s normal function in such a way as to cause permanent or reversible damage to neural tissue, this is known as neurotoxicity.
One of the main causes of toxic responses, which may lead to the failure of many organs, is systemic toxicity, and this is fueling the expansion of this market even more. While systemic circulation is the preferred mode of drug delivery, it also plays a major role in toxic responses that may cause organ failure. Other significant applications that are expected to grow rapidly include renal toxicity and hepatotoxicity, both of which have seen more active research and studies conducted in these fields.
By Method Analysis
The cellular Assay method segment is Dominant in the ADME Toxicology Testing Market.
Based on the method, the market is divided into Cellular Assay, Biochemical Assay, In Silica, and Ex-vivo. The Cellular Assay dominates the market with 45% of the market share. The highly precise, exact, and natural cell assay methods are aiding this segment’s growth.
Cell-based physiological parameter tests act as a bridge between laboratories and clinics, making human testing less risky without necessarily eliminating clinical trials. Furthermore, these tests reduce animal needs for testing considerably – something expected to play an increasingly significant role in increasing awareness around animal rights issues.
Key Market Segments
Based on Technology
- Cell Culture
- High Throughput Screening
- Molecular Imaging
- OMICS Technology
Based on Application
- Systemic Toxicity
- Renal Toxicity
- Hepatotoxicity
- Neurotoxicity
- Other Application
Based on Method
- Cellular Assay
- Biochemical Assay
- In Silica
- Ex-vivo
Drivers
The pharmaceutical industry’s rapid expansion is one of the primary drivers of demand for ADME toxicology testing services. As drug development activities increase, so too does the demand for these tests. ADME testing provides essential data on the safety and effectiveness of drug compounds, which is necessary for gaining regulatory approval. With an increasing trend towards personalized medicine – creating medications tailored specifically for individual patient needs – ADME testing plays a vital role in this process.
ADME toxicology testing is essential in developing personalized medicines, providing insights into how drugs will be metabolized and excreted in specific patient populations. Government regulatory organizations such as the FDA and EMA require drug developers to conduct extensive ADME toxicology testing on new drug candidates before they can be approved for clinical trials. This regulatory requirement is propelling growth in the ADME toxicology testing market. Technological advances have made ADME toxicology testing more efficient, precise, and cost-effective.
For instance, in silico modeling and simulation techniques have revolutionized the drug development process by eliminating animal testing requirements and speeding up the identification of drug candidates. With growing public awareness of the potential hazards associated with pharmaceuticals, there is an increasing demand for safe and effective drugs. ADME toxicology testing helps guarantee that drug compounds undergo thorough evaluation for safety and effectiveness before being released into the market.
The non-clinical safety assessment segment is the largest application area of ADME toxicology testing, as it evaluates the safety of drugs, chemicals, and other substances before they are administered to humans. As demand for non-clinical safety assessments grows, so too will the ADME toxicology testing market.
Restraints
ADME toxicology testing can be expensive, especially for small and medium-sized companies with tight budgets. The high price tag may deter some businesses from investing in it, potentially slowing down the market’s growth. In addition, lack of standardization in ADME toxicology testing can be a significant challenge, leading to inconsistent results and difficulty comparing data across different studies.
Unfortunately, regulatory approval can be lengthy and tedious for drug candidates, potentially slowing down drug development. Animal testing for ADME toxicology has raised ethical issues, prompting an increasingly popular shift to alternative methods such as in silico modeling and simulation. However, these methods are still not widely adopted, and further research is necessary to prove their efficiency and effectiveness.
Technological advances have made ADME toxicology testing more efficient, accurate, and cost-effective. For instance, in silico modeling and simulation techniques have revolutionized the drug development process by eliminating animal testing requirements and speeding up the identification of drug candidates. With growing public awareness of the potential hazards associated with pharmaceuticals, there is an increasing need for safe and effective drugs. ADME toxicology testing helps guarantee that drug compounds undergo rigorous evaluation for safety and efficacy before they are released onto the market.
Opportunity
In vitro testing is becoming increasingly popular, which involves testing drug compounds on cells or tissues in a laboratory setting. In vitro testing offers several advantages over traditional animal testing such as reduced costs, faster results, and improved ethical considerations. Recent advances in ADME modeling have enabled scientists to more accurately predict how drug compounds will behave inside humans, leading to improved efficiency during drug development and lower costs associated with animal testing.
Pharmacogenomics is the study of how an individual’s genes influence their response to drugs. ADME toxicology testing plays a significant role in this field, providing insights into how drugs will be metabolized and excreted within specific patient populations. This has significant ramifications for the development of personalized medicine. Emerging markets such as Asia-Pacific, Latin America, and the Middle East are expected to offer significant growth prospects for ADME toxicology testing market players.
This trend can be attributed to an increasing need for healthcare services, rising income levels, and government initiatives to improve healthcare infrastructure. Collaborations and partnerships among pharmaceutical companies, contract research organizations (CROs), and academic institutions are fueling innovation in the ADME toxicology testing market. These endeavors aim to develop new testing methods, boost accuracy levels, and cut down on costs associated with drug development.
Trends
One of the key trends in ADME (Absorption, Distribution, Metabolism, and Excretion) toxicology testing is the increasing adoption of in silico modeling and simulation techniques. In silico modeling is the practice of using computer models to simulate drug compounds’ actions within the human body. This technique enables researchers to evaluate drug candidates more efficiently and cost-effectively, eliminating the need for animal testing and speeding up drug identification.
Another important trend in medical research is the rising importance of personalized medicine. As our understanding of genetics advances, personalized medicine is becoming an increasingly important field. ADME toxicology testing is essential in developing personalized medicine, providing insights into how drugs will be metabolized and excreted in specific patient populations. Furthermore, demand for non-clinical safety assessment has grown rapidly in the ADME toxicology testing market. Non-clinical safety assessment involves testing the safety of drugs, chemicals, and other substances before they are administered to humans.
This demand is being driven by increased public awareness of potential hazards associated with pharmaceuticals and the need to guarantee safety for new drug candidates. Another trend in ADME toxicology testing is an increasing reliance on high-throughput screening (HTS) techniques. HTS involves screening large numbers of compounds simultaneously to identify potential drug candidates quickly. This technique has the potential to reduce the time and expense associated with drug development while increasing success rates for drug candidates.
Regional Analysis
The largest share in the market will be dominated by North America, with 49% of the market share.
North America currently dominates the ADME toxicology testing market due to the presence of major players and the increasing demand for advanced toxicology testing services in this region. Furthermore, with established healthcare infrastructure, supportive government initiatives, and high awareness about toxicology testing among consumers, North America is on track to become a major growth area in this sector.
Latin America and the Middle East/Africa regions are expected to experience substantial growth in the ADME toxicology testing market due to increased investments into healthcare infrastructure, rising government initiatives for drug discovery and development, as well as growing awareness regarding its significance among these regions.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the competitive landscape for ADME toxicology testing, detailed information about each competitor is included. This includes financials and revenue generated, market potential, R&D investments, and new market initiatives as well as global presence with production sites and facilities, production capacities, strengths and weaknesses of each firm, product launch strategy, product width/breadth, and application dominance. Ultimately this data only pertains to companies’ focus on ADME toxicity testing.
Market Key Players
- Thermo Fisher Scientific Inc
- Promega Corporation
- Agilent Technologies Inc
- Dassault Systèmes
- Beckman Coulter Inc
- Catalent Inc
- Charles River Laboratories
- Eurofins Scientific
- General Electric
- Merck KGaA
- Miltenyi Biotec
- Bio-Rad Laboratories Inc
- BioIVT LLC
- Catalent Inc
- AbbVie
- Accelrys Inc
- Cyprotex Plc (Evotec AG)
- Molecular Discovery Ltd.
- Perkinelmer Inc.
- Other Key players
Recent Developments
- Thermo Fisher Scientific Inc (April 2024): Thermo Fisher Scientific Inc. launched the latest version of their CellInsight CX7 High-Content Screening Platform, enhancing ADME-toxicology testing with improved imaging capabilities, advanced data analysis software, and increased throughput for more efficient and accurate toxicity assessments.
- Promega Corporation (June 2024): Promega Corporation introduced the P450-Glo™ Assays, a series of luminescent assays designed for the rapid and accurate evaluation of cytochrome P450 enzyme activity, significantly improving the efficiency and reliability of ADME-toxicology testing in drug discovery processes.
- Agilent Technologies Inc (May 2024): Agilent Technologies Inc. acquired BioTek Instruments, a leader in microplate instrumentation and bioassay technologies, to enhance their portfolio of ADME-toxicology testing solutions, providing more comprehensive and integrated testing capabilities to pharmaceutical and biotechnology industries.
- Dassault Systèmes (March 2024): Dassault Systèmes merged with BIOVIA, a leading provider of scientific software solutions, to strengthen their ADME-toxicology testing offerings, integrating advanced simulation and modeling tools for more precise and predictive toxicology assessments.
- Beckman Coulter Inc (February 2024): Beckman Coulter Inc. launched the Vi-CELL BLU Cell Viability Analyzer, designed to provide accurate and automated cell viability and cytotoxicity testing, enhancing the efficiency and reliability of ADME-toxicology assessments in drug development.
- Catalent Inc (January 2024): Catalent Inc. acquired BioDuro-Sundia, a contract research, development, and manufacturing organization, to expand their ADME-toxicology testing capabilities, offering more comprehensive and integrated solutions for drug development and safety testing.
Report Scope
Report Features Description Market Value (2023) USD 9.3 Billion Forecast Revenue (2032) USD 26.2 Billion CAGR (2023-2032) 12.5% Base Year for Estimation 2022 Historic Period 2016-2021 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered Based on Technology(Cell Culture, High Throughput Screening, Molecular Imaging, OMICS Technology) Based on Application(Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Other Application) Based on Method, (Cellular Assay, Biochemical Assay, In Silica, Ex-vivo) Regional Analysis North America – The US, Canada,&Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, &Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, &Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, &Rest of MEA Competitive Landscape Thermo Fisher Scientific Inc, Promega Corporation, Agilent Technologies Inc, Dassault Systèmes, Beckman Coulter Inc, Catalent Inc, Charles River Laboratories, Eurofins Scientific, General Electric, Merck KGaA, Miltenyi Biotec, Bio-Rad Laboratories Inc, BioIVT LLC, Catalent Inc, AbbVie, Accelrys Inc, Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., Other Key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What does ADME stand for in toxicology testing?ADME stands for Absorption, Distribution, Metabolism, and Excretion. These processes are studied to understand the pharmacokinetics of a drug and its potential toxicological effects.
How big is the ADME-Toxicology Testing Market?The global ADME-Toxicology Testing Market size was estimated at USD 9.3 Billion in 2023 and is expected to reach USD 26.2 Billion in 2032.
What is the ADME-Toxicology Testing Market growth?The global ADME-Toxicology Testing Market is expected to grow at a compound annual growth rate of 12.5%. From 2024 To 2033
Who are the key companies/players in the ADME-Toxicology Testing Market?Some of the key players in the ADME-Toxicology Testing Markets are Thermo Fisher Scientific Inc, Promega Corporation, Agilent Technologies Inc, Dassault Systèmes, Beckman Coulter Inc, Catalent Inc, Charles River Laboratories, Eurofins Scientific, General Electric, Merck KGaA, Miltenyi Biotec, Bio-Rad Laboratories Inc, BioIVT LLC, Catalent Inc, AbbVie, Accelrys Inc, Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., Other Key players
Why is ADME-toxicology testing important?ADME-toxicology testing is crucial for evaluating the safety and efficacy of new drugs. It helps in identifying potential toxicities early in the drug development process, thereby reducing the risk of adverse effects in humans.
What are the primary methods used in ADME-toxicology testing?Common methods include in vitro assays, in vivo animal studies, high-throughput screening, and computational modeling. These methods assess how a drug is absorbed, distributed, metabolized, and excreted by the body.
Which industries primarily use ADME-toxicology testing?The pharmaceutical and biotechnology industries are the primary users. These tests are also essential in chemical, cosmetic, and food industries for safety assessments.
 ADME-Toxicology Testing MarketPublished date: July 2024add_shopping_cartBuy Now get_appDownload Sample
ADME-Toxicology Testing MarketPublished date: July 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Thermo Fisher Scientific Inc
- Promega Corporation
- Agilent Technologies Inc
- Dassault Systèmes
- Beckman Coulter Inc
- Catalent Inc
- Charles River Laboratories
- Eurofins Scientific
- General Electric
- Merck KGaA
- Miltenyi Biotec
- Bio-Rad Laboratories Inc
- BioIVT LLC
- Catalent Inc
- AbbVie
- Accelrys Inc
- Cyprotex Plc (Evotec AG)
- Molecular Discovery Ltd.
- Perkinelmer Inc.
- Other Key players









