Real World Evidence (RWE) Solutions Market Based on Component-(Services, Data Sets, Clinical Settings Data and More) Application-(Drug Development & Approvals, Medical Device Development & Approvals, Post Market Safety & Adverse Events Monitoring)Therapeutic Area-(Oncology, Cardiology, Neurology, Other Therapeutic Areas)End-user-(Healthcare Companies, Healthcare Payers, Healthcare Providers, Other End-Users) by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2023-2033
- Published date: Nov 2023
- Report ID: 83875
- Number of Pages: 255
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
The Global Real-World Evidence/RWE Solutions Market size is expected to be worth around USD 5.8 Billion by 2033 from USD 2.7 Billion in 2023, growing at a CAGR of 7.9% during the forecast period from 2023 to 2032.
Every day, new devices and devices with new features are launched. The plethora of Real World Evidence (RWE) that has been collected to help us make informed decisions on these devices has become essential.
Real-world evidence or RWE is the use of data collected from large groups of patients in real-world settings to support medical decision-making. The “real world” refers to all areas outside a traditional clinical trial setting which includes different patient populations, treatments, and dosing schedules. RWE is increasingly being used as an effective tool to generate evidence for healthcare stakeholders to support treatment decisions.
With the growing number of patients and limited medical personnel, the cost of healthcare is continually on the rise. In order to stay competitive in this market, healthcare providers must find new ways to keep costs down and work towards more efficient ways of reaching their goals. Real-world evidence solutions help hospitals receive insights on how to improve processes in order to decrease personnel hours spent on routine tasks, which in turn decreases overall costs.

Key Takeaways
- Market Growth: Real-World Evidence/RWE Solutions Market size is expected to be worth around USD 5.8 Billion by 2033 from USD 2.7 Billion in 2023
- Component Analysis: The services segment accounted for the maximum revenue share of over 55.9% in 2023
- Application Analysis: The segment of drug development and approvals was the most profitable with a revenue share, which was more than 29.6% in 2023
- End-User Analysis: The segment of healthcare companies has the highest revenue share of 32.6% by 2023
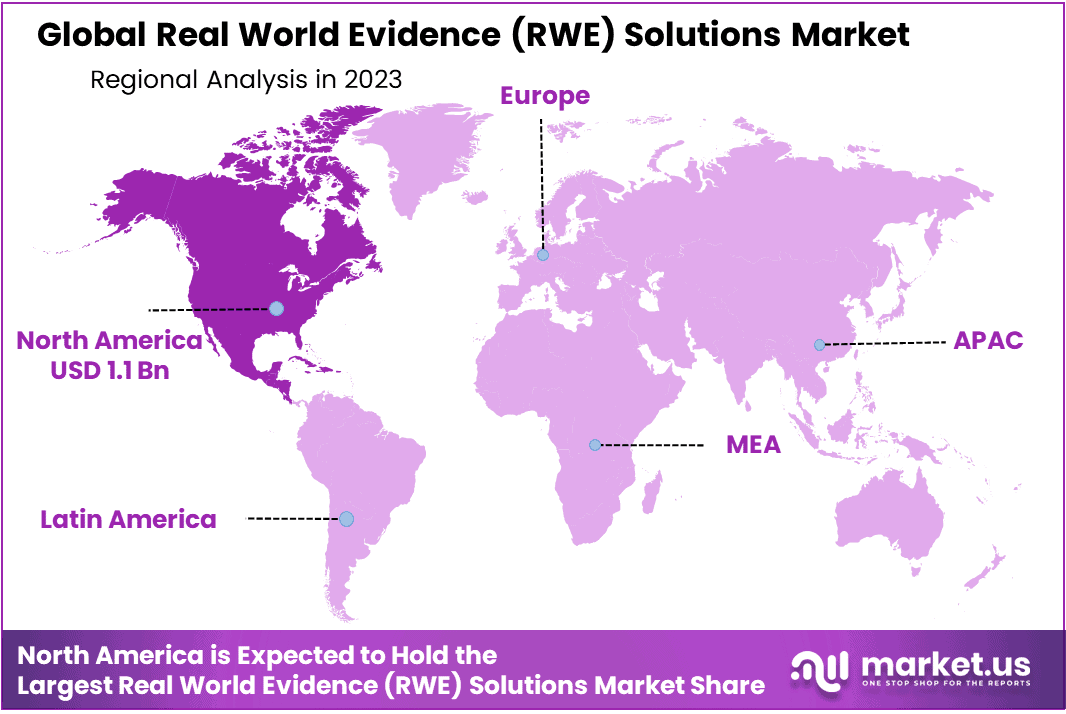
Regional Analysis:North America accounted for the largest revenue share of over 41.5.0% in 2023 and holding a USD 1.1 Billion - Diverse Data Sources: RWE solutions aggregate data from various sources, including electronic health records, claims data, social media, wearable devices, and other healthcare databases.
- Technology Advancements: Advances in technology, such as big data analytics, machine learning, and artificial intelligence, have played a significant role in the development of RWE solutions.
Component Analysis
The services segment accounted for the maximum revenue share of over 55.9% in 2023. The growth is attributed to the high adoption of real-world services by pharmaceutical & biotechnology companies as well as healthcare providers. The segment is also anticipated to witness the fastest CAGR during the forecast period.
The consulting services sector is expected to grow at a substantial rate over the forecast time. Because of the growing need for consultants who can provide services to speed up the process of developing drugs and speed up the clinical trials process in order to obtain rapid approval for market.
Application Analysis
The segment of drug development and approvals was the most profitable with a revenue share, which was more than 29.6% in 2023. Solutions for real-world evidence enable healthcare and pharmaceutical providers as well as payers to have efficient operation management and speed up the process of developing drugs and approval. This boosts the growth of markets.
The segment of drug development approvals is expected to grow significantly over the forecast time frame due to the increase in number of chronic illnesses and the increasing demand for new treatments for drug development and medical devices. These boost the demand for drugs approvals based on authentic evidence-based solutions.
End-User Analysis
The segment of healthcare companies has the highest revenue share of 32.6% by 2023. This growth is due to the increasing importance of RWE studies during the process of approving drugs as well as evaluating the effectiveness of drugs in real-world situations and preventing recalls of drugs.
Healthcare payers are anticipated to experience significant market growth in the coming years due to the growing numbers of providers and the increase in the acceptance of the most advanced technological real-world evidence tools

Key Market Segments
Component
- Services
- Data Sets
- Clinical Settings Data
- Claims Data
- Pharmacy Data
- Patient-Powered Data
Application
- Drug Development & Approvals
- Medical Device Development & Approvals
- Reimbursement/Coverage & Regulatory Decision Making
- Post Market Safety & Adverse Events Monitoring
Therapeutic Area
- Oncology
- Cardiology
- Neurology
- Diabetes
- Psychiatry
- Respiratory
- Other Therapeutic Areas
End-user
- Healthcare Companies
- Healthcare Payers
- Healthcare Providers
- Other End-Users
Driver
Increasing Adoption by Pharmaceutical and Biotechnology Companies
One major driver propelling the Real World Evidence (RWE) Solutions Market is the escalating adoption of these solutions by pharmaceutical and biotechnology companies. The industry’s recognition of the value of real-world data in assessing drug performance outside of controlled clinical trials is driving the demand for RWE solutions. Companies are leveraging these solutions to gain insights into the real-world effectiveness and safety of their products, contributing to informed decision-making in drug development and market access.
Trend
Integration of Advanced Technologies like Artificial Intelligence
A notable trend shaping the RWE Solutions Market is the integration of advanced technologies, particularly artificial intelligence (AI). The use of AI in analyzing vast and diverse real-world datasets enables more efficient and precise extraction of meaningful insights. This trend enhances the capabilities of RWE solutions, providing stakeholders with a deeper understanding of treatment outcomes, patient experiences, and healthcare utilization patterns.
Restraint
Data Privacy and Security Concerns
A significant restraint impacting the market is the growing concern over data privacy and security. The utilization of real-world data involves handling sensitive patient information, raising apprehensions about unauthorized access and potential breaches. Addressing these concerns and ensuring compliance with stringent data protection regulations pose challenges to the widespread adoption of RWE solutions.
Opportunity
Expanding Applications in Healthcare Decision-Making
An exciting opportunity lies in the expanding applications of RWE solutions in healthcare decision-making. Beyond their traditional use in drug development, RWE solutions are increasingly employed in health policy, treatment guidelines, and patient care decisions. The evolving role of real-world evidence in shaping broader healthcare strategies opens up avenues for market growth and encourages stakeholders to explore diverse applications across the healthcare landscape.
Regional Analysis
North America accounted for the largest revenue share of over 41.5.0% in 2023 and holding a USD 1.1 Billion value for the Real World Evidence (RWE) Solutions Market. The large share of the North American region is credited to the presence of key players in the U.S. Rising number of RWE service providers and favorable government regulations in the region is also expected to contribute to the market growth.
Asia-Pacific was the second largest contributor to the market in 2023, and is expected to register the fastest CAGR during the forecast period, owing to rise in geriatric population and increase in the prevalence of cancer and other chronic diseases across the region.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global market is extremely competitive and divided. Market players are implementing strategic initiatives like launch and development of new products expanding distribution networks and global reach through partnership and subsidiaries.
Market Key Players
- IQVIA
- IBM
- PPD, Inc.
- Parexel International Corporation
- PerkinElmer Inc.
- Icon Plc
- Oracle
- Syneos Health
- Cegedim Health Data
- Medpace
Recent Development
- January 2023 Advanced analytics and data solutions for healthcare organizations to make informed decisions regarding patient care and drug development decisions. The acquisition will expand IQVIA’s real world data capabilities and assist organizations make informed decisions related to patient care and drug development decisions.
- March 2023 Real-World Evidence Coalition (RWEC) published a white paper outlining potential benefits of RWE use for medical device development and regulation, calling for closer cooperation between industry, regulators, patient advocacy groups, and patients themselves to accelerate its use within this arena.
- July 2023 CMS published a proposed rule which would require Medicare Advantage plans to use real world evidence (RWE) in their value-based care programs and increase use of RWE within them, improving patient care and outcomes overall.
Report Scope
Report Features Description Market Value (2023) USD 2.7 Billion Forecast Revenue (2033) USD 5.8 Billion CAGR (2024-2033) 7.9% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered Component-(Services, Data Sets, Clinical Settings Data, Claims Data, Pharmacy Data, Patient-Powered Data) Application-(Drug Development & Approvals, Medical Device Development & Approvals, Reimbursement/Coverage & Regulatory Decision Making, Post Market Safety & Adverse Events Monitoring)Therapeutic Area-(Oncology, Cardiology, Neurology, Diabetes, Psychiatry, Respiratory, Other Therapeutic Areas)End-user-(Healthcare Companies, Healthcare Payers, Healthcare Providers, Other End-Users) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape IQVIA, IBM, PPD, Inc., Parexel International Corporation, PerkinElmer Inc., Icon Plc, Oracle, Syneos Health, Cegedim Health Data, Medpace Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Real World Evidence (RWE) Solutions MarketPublished date: Nov 2023add_shopping_cartBuy Now get_appDownload Sample
Real World Evidence (RWE) Solutions MarketPublished date: Nov 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- IQVIA
- IBM
- PPD, Inc.
- Parexel International Corporation
- PerkinElmer Inc.
- Icon Plc
- Oracle
- Syneos Health
- Cegedim Health Data
- Medpace









