Global Juvenile Idiopathic Arthritis Diagnostic Market By Test (Blood Tests, ESR, Anti-Nuclear Antibody, C-Reactive Protein and Others), By End-user (Hospitals and Research Laboratories), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Dec 2025
- Report ID: 171281
- Number of Pages: 359
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
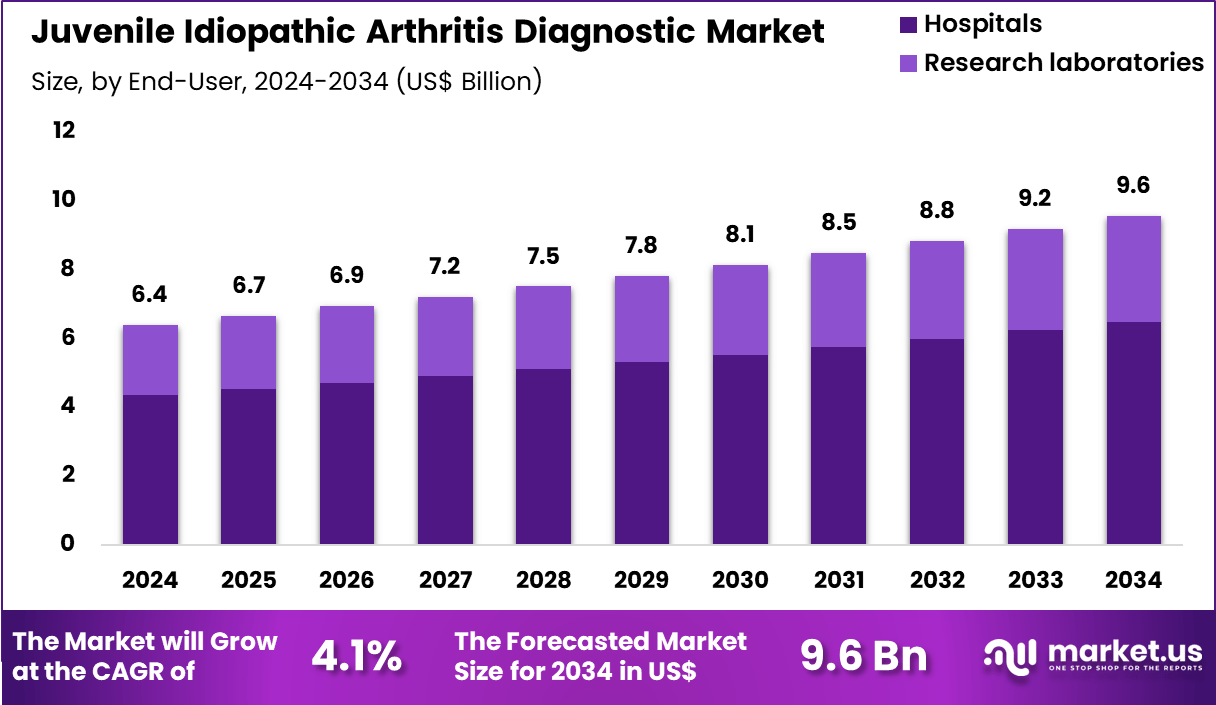
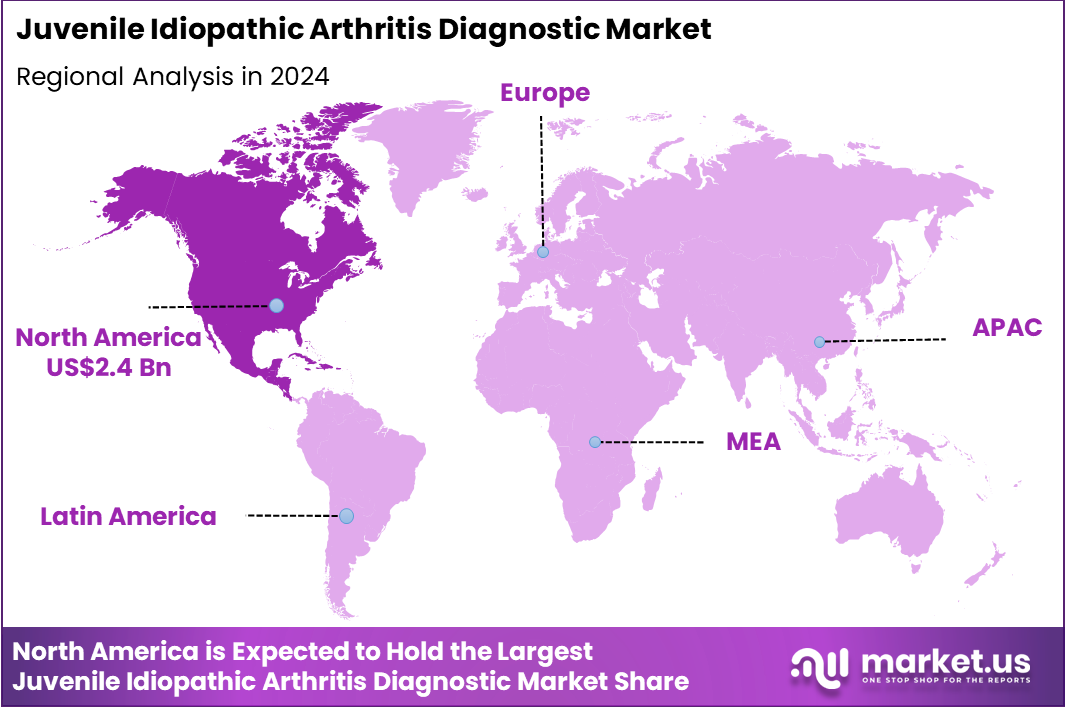
The Global Juvenile Idiopathic Arthritis Diagnostic Market size is expected to be worth around US$ 9.6 billion by 2034 from US$ 6.4 Billion in 2024, growing at a CAGR of 4.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 36.9% share with a revenue of US$ 2.4 Billion.
Increasing awareness of autoimmune disorders in pediatric populations propels the Juvenile Idiopathic Arthritis Diagnostic market, as healthcare providers emphasize early intervention to mitigate long-term joint damage and improve quality of life. Diagnostic companies innovate with serological panels that detect rheumatoid factor and antinuclear antibodies alongside inflammatory markers like C-reactive protein.
These tools enable initial screening in children presenting with persistent joint swelling, differentiation of oligoarticular from polyarticular subtypes for tailored therapy selection, and monitoring of treatment response in enthesitis-related arthritis cases. Opportunities arise from integrating genetic testing to identify HLA-B27 associations, facilitating prognostic stratification and family counseling.
Juvenile Idiopathic Arthritis Diagnostic Market Size Recent guidelines highlight the role of ferritin and advanced imaging in confirming Still’s disease, including systemic juvenile idiopathic arthritis; on November 29, 2025, EULAR and PReS jointly issued updated recommendations that position elevated ferritin levels and modalities like MRI as essential for diagnosis and early detection of complications such as Macrophage Activation Syndrome. This framework strengthens clinical protocols and drives demand for comprehensive diagnostic suites.
Growing adoption of non-invasive imaging technologies accelerates the Juvenile Idiopathic Arthritis Diagnostic market, as rheumatologists leverage ultrasound and MRI to visualize synovitis and cartilage erosion without radiation exposure. Manufacturers develop portable ultrasound devices equipped with Doppler capabilities that quantify vascularity in affected joints.
Applications encompass baseline assessments in systemic-onset cases to guide interleukin-1 inhibitor initiation, progression tracking in psoriatic arthritis to prevent skin-joint discordance, and therapeutic efficacy evaluation during methotrexate trials. Emerging opportunities include AI-enhanced image analysis that automates synovial hypertrophy scoring for objective follow-up.
Biotechnology firms increasingly incorporate these modalities into multidisciplinary care models that combine imaging with biomarker assays. This technological synergy enhances diagnostic accuracy and supports remote monitoring in pediatric outpatient settings.
Rising integration of biomarker discovery invigorates the Juvenile Idiopathic Arthritis Diagnostic market, as researchers identify novel proteins like S100A8/A9 for subtype-specific profiling and disease flare prediction. Laboratories deploy multiplex immunoassay platforms that simultaneously measure calprotectin and matrix metalloproteinases from synovial fluid or serum.
These assays facilitate risk assessment in undifferentiated arthritis to expedite classification, prognostic modeling in oligoarticular disease for uveitis screening, and longitudinal surveillance in polyarticular rheumatoid factor-negative cases under biologic therapy. Precision diagnostics create opportunities for companion tests that personalize JAK inhibitor dosing based on cytokine signatures. Pharmaceutical developers actively collaborate on validation studies to embed these biomarkers into regulatory pathways. This molecular focus positions advanced testing as a cornerstone of proactive juvenile idiopathic arthritis management.
Key Takeaways
- In 2024, the market generated a revenue of US$ 6.4 Billion, with a CAGR of 4.1%, and is expected to reach US$ 9.6 Billion by the year 2034.
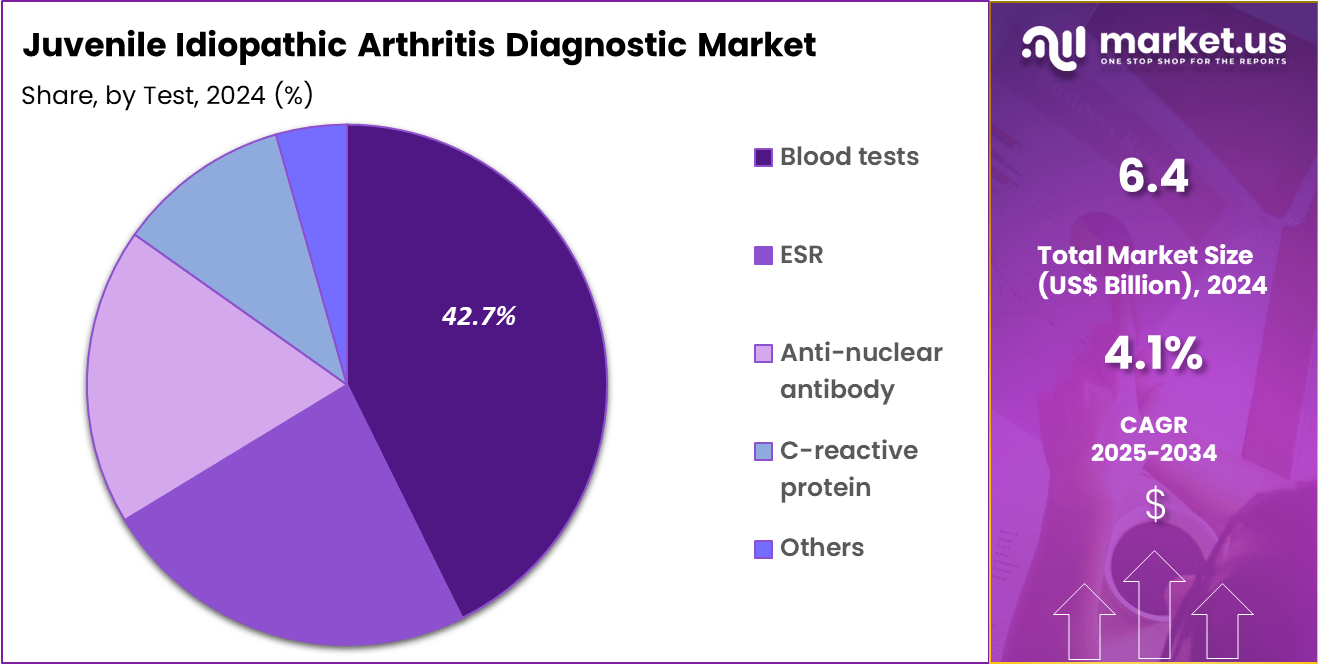
- The test segment is divided into blood tests, ESR, anti-nuclear antibody, c-reactive protein and others, with blood tests taking the lead in 2023 with a market share of 42.7%.
- Considering end-user, the market is divided into hospitals and research laboratories. Among these, hospitals held a significant share of 67.9%.
- North America led the market by securing a market share of 36.9% in 2024.
Test Analysis
Blood tests, holding 42.7%, are expected to dominate because they serve as the primary method for identifying inflammatory biomarkers associated with juvenile idiopathic arthritis in children. Physicians rely on tests such as complete blood count and rheumatoid factor evaluation to differentiate JIA from infections or traumatic injuries. Rising awareness about the importance of early detection strengthens the preference for blood-based evaluations in pediatric settings.
The growing number of autoimmune conditions among children increases the need for accurate and routine monitoring tools. Automated laboratory systems improve result reliability and shorten reporting time, which enhances treatment planning.
Parents increasingly seek timely diagnosis to prevent long-term joint damage in children, driving test utilization further. Public health initiatives promoting pediatric rheumatology referral pathways support the expansion of blood testing in clinical practices. These factors keep blood tests anticipated to remain the dominant diagnostic method in the JIA diagnostics market.
End-User Analysis
Hospitals, holding 67.9%, are projected to dominate as they act as the first point of care for children showing persistent joint symptoms requiring thorough evaluation. Pediatric rheumatologists in hospitals rely on advanced diagnostic resources such as serology, imaging, and immunological analysis to confirm JIA.
The ability to offer multidisciplinary care, including orthopedic and rehabilitation support, strengthens hospital dependency for early disease management. Increasing healthcare accessibility encourages timely hospitalization for accurate diagnosis and continued monitoring of disease progression. The availability of modern laboratory infrastructure inside hospitals supports precise result interpretation and treatment planning.
Rising investments in pediatric specialty centers improve diagnostic capabilities across regions. Longer-term patient follow-ups and frequent re-assessment needs ensure hospitals remain key locations for testing. These factors keep hospitals expected to remain the leading end-user segment in this market.
Key Market Segments
By Test
- Blood Tests
- ESR
- Anti-Nuclear Antibody
- C-Reactive Protein
- Others
By End-user
- Hospitals
- Research Laboratories
Drivers
The need for early and accurate diagnosis is driving the market
Juvenile idiopathic arthritis represents the most common chronic rheumatic disease in children, necessitating prompt diagnostic interventions to mitigate joint damage and functional impairment. Early identification allows for timely initiation of disease-modifying therapies, which can significantly alter disease trajectories and enhance quality of life.
The diagnostic process typically involves a combination of clinical evaluation, laboratory tests such as antinuclear antibody screening, and imaging modalities like ultrasound to detect synovial inflammation. This multifaceted approach underscores the market’s expansion as healthcare providers seek comprehensive tools to confirm diagnoses swiftly. Moreover, the variability in JIA subtypes, ranging from oligoarticular to systemic forms, demands specialized diagnostic strategies tailored to each presentation.
Public health campaigns by organizations like the National Institutes of Health have heightened parental and clinician awareness, prompting increased screening in primary care settings. Consequently, diagnostic laboratories are experiencing heightened demand for high-sensitivity assays to differentiate JIA from infectious or neoplastic mimics.
The integration of multidisciplinary teams, including rheumatologists and radiologists, further propels the need for advanced diagnostic infrastructure. As pediatric populations grow, the cumulative burden of undiagnosed cases amplifies the urgency for scalable diagnostic solutions. Ultimately, this driver fosters innovation in point-of-care testing, positioning the market for sustained growth amid evolving pediatric rheumatology practices.
Restraints
The heterogeneity of clinical presentations is restraining the market
The diverse subtypes of juvenile idiopathic arthritis, encompassing oligoarticular, polyarticular, systemic, psoriatic, enthesitis-related, and undifferentiated forms, complicate uniform diagnostic protocols. This variability often leads to misclassification or delayed confirmation, as initial symptoms may mimic other pediatric conditions like leukemia or Lyme disease.
Laboratory markers such as rheumatoid factor and anti-cyclic citrullinated peptide antibodies exhibit low sensitivity in many JIA cases, limiting their standalone utility. Imaging findings, while supportive, require expert interpretation to distinguish active synovitis from growth-related changes in children. The absence of a single gold-standard test exacerbates diagnostic uncertainty, particularly in early disease stages where symptoms are subtle.
Resource constraints in non-specialized centers hinder access to advanced tools like magnetic resonance imaging, perpetuating inequities in care delivery. Moreover, evolving consensus criteria from bodies like the International League of Associations for Rheumatology demand ongoing updates to diagnostic workflows, straining healthcare systems.
Patient-specific factors, including age at onset and genetic predispositions, further confound standardization efforts. These challenges result in prolonged time-to-diagnosis, averaging several months in some cohorts, which correlates with poorer prognostic outcomes. Collectively, this restraint curtails the widespread adoption of JIA diagnostics, impeding market penetration and innovation.
Opportunities
The integration of artificial intelligence in imaging analysis is creating growth opportunities
Artificial intelligence algorithms enhance the precision of ultrasound and MRI interpretations for detecting subclinical joint involvement in juvenile idiopathic arthritis. By automating synovial hypertrophy quantification, AI reduces inter-observer variability and accelerates reporting times in busy clinical environments.
Machine learning models trained on electronic health records achieve positive predictive values up to 97% for JIA diagnosis using International Classification of Diseases codes, as demonstrated in multi-institutional validations. This capability supports risk stratification, enabling personalized monitoring plans that optimize resource allocation. Furthermore, AI facilitates the analysis of longitudinal data to predict disease flares, informing proactive therapeutic adjustments.
Collaborative initiatives between academic centers and technology developers are yielding cloud-based platforms for real-time diagnostic support in remote settings. The scalability of these tools addresses the shortage of pediatric rheumatologists, broadening access to expert-level assessments. Integration with wearable sensors for activity tracking complements AI-driven imaging, offering holistic disease surveillance.
Regulatory endorsements from agencies like the Food and Drug Administration for AI-assisted diagnostics signal a maturing ecosystem ripe for investment. In summary, these advancements unlock opportunities for cost-effective, equitable diagnostic solutions, propelling market expansion through enhanced clinical efficacy.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic forces energize the juvenile idiopathic arthritis diagnostic market as expanding healthcare budgets and greater awareness of pediatric rheumatology push clinics and hospitals to adopt advanced imaging, biomarker tests, and genetic screening for earlier, more precise interventions. Leading manufacturers swiftly innovate with portable ultrasound devices and multiplex assays, capitalizing on the surge in demand for non-invasive tools that enhance patient outcomes in growing populations.
Persistent inflation and sluggish economic recoveries, however, compress operational budgets for diagnostic centers, compelling providers to defer equipment purchases and limit access in underfunded regions. Geopolitical tensions, including U.S.-China trade disputes and regional conflicts, disrupt global flows of critical components like reagents and scanners, generating supply shortages and escalating procurement challenges for international suppliers.
Current U.S. tariffs impose hefty duties on imported medical devices and kits, driving up costs for essential arthritis diagnostics and straining margins for distributors reliant on overseas production. These tariffs provoke retaliatory restrictions in foreign markets that impede U.S. exports and slow joint research initiatives on novel biomarkers. Nevertheless, the policies accelerate investments in domestic manufacturing hubs and localized innovation, forging resilient supply networks that promise enhanced stability and broader market reach for the long term.
Latest Trends
The development of machine learning-based diagnostic models is a recent trend
In 2024, researchers introduced a novel machine learning model utilizing transcriptomic data to identify key biomarkers for systemic juvenile idiopathic arthritis, marking a shift toward genomics-informed diagnostics. This random forest algorithm incorporates genes such as ALDH1A1 and CEACAM1 to differentiate disease states from healthy controls with improved granularity.
The model’s design leverages public genetic databases, promoting reproducibility and accessibility for global research consortia. By predicting inactive disease status at 18 months post-diagnosis, it aids in tailoring long-term management strategies. This trend aligns with broader efforts in pediatric rheumatology to harness computational tools for precision medicine. Early validations highlight its superiority over traditional clinical determinants in prognostic accuracy.
Adoption in clinical trials is accelerating, with implications for subtype-specific screening protocols. Collaborative platforms are emerging to standardize data inputs, ensuring model robustness across diverse populations. This innovation addresses longstanding gaps in biomarker discovery, fostering confidence in molecular diagnostics. Overall, the 2024 rollout exemplifies a pivotal evolution in JIA diagnostics, emphasizing data-driven paradigms for future therapeutic decisions.
Regional Analysis
North America is leading the Juvenile Idiopathic Arthritis Diagnostic Market
North America accounted for 36.9% of the overall market in 2024, and the region experienced continued growth as pediatric rheumatology networks strengthened early-detection pathways for autoimmune joint disorders in children. Diagnostic centers expanded access to ANA, RF, and HLA-B27 testing, along with MRI for early visualization of synovial inflammation.
Increased awareness among pediatricians about fatigue, persistent joint swelling, and limping in children led to more timely referrals for autoimmune evaluation. Clinical use of biomarker panels improved differentiation of JIA subtypes, supporting more accurate and earlier diagnosis.
The CDC estimates that nearly 300,000 children in the United States are affected by arthritis, including juvenile idiopathic arthritis (CDC – Arthritis Data & Statistics, 2023), and this large pediatric burden drove demand for diagnostic services. Expanding insurance coverage for advanced rheumatology diagnostics further supported market adoption. These combined developments helped strengthen the diagnostic market’s position in North America during 2024.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to see strong growth during the forecast period as regional healthcare systems expand pediatric autoimmune-disease screening for earlier diagnosis and intervention. Hospitals invest in advanced laboratory analyzers and imaging technologies to detect joint inflammation and immune dysregulation in children.
Rising clinical recognition of JIA symptoms improves referral practices across urban and semi-urban pediatric care. Public-health programs increasingly highlight childhood musculoskeletal disorders, prompting earlier evaluation by specialists. Workforce expansion in pediatric rheumatology improves access to diagnostic expertise, particularly in China, Japan, and India.
The Japanese Society of Pediatric Rheumatology reports approximately 10,000 children living with JIA in Japan (2023 registry summary), illustrating the growing clinical need for standardized testing. Manufacturers also strengthen regional distribution of autoimmune-diagnostic reagents. These factors collectively position Asia Pacific for sustained growth over the coming years.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Leading players in pediatric rheumatology diagnostics focus on broader autoantibody and inflammatory-marker panels, combine these with imaging and genetic markers, and position integrated workups as the standard for early detection and risk stratification. They invest in high-throughput immunoassay platforms and advanced analytics so hospital labs and reference centers process complex pediatric profiles quickly and with consistent quality.
Commercial teams strengthen growth by partnering with children’s hospitals, specialty clinics, and research consortia to co-develop algorithms that link lab results with clinical scoring tools. Management prioritizes geographic expansion into regions where awareness of childhood rheumatic diseases rises and where payers start to reimburse comprehensive immune and inflammatory testing.
Marketing and medical-affairs units run education initiatives for pediatricians and rheumatologists that highlight the clinical and economic value of structured diagnostic workups, which supports repeat testing and long-term customer relationships.
Quest Diagnostics exemplifies this approach as it operates one of the largest diagnostic networks in the Americas, offers extensive autoimmune and inflammatory test menus relevant to pediatric rheumatology, and leverages its logistics, digital reporting platforms, and specialist consult services to anchor its position as a preferred partner in complex childhood arthritis evaluation.
Top Key Players
- Johnson & Johnson Services, Inc.
- Novartis International AG
- Bristol-Myers Squibb Company
- Zydus Cadila
- Genentech, Inc.
- Latona Life Sciences
- AbbVie
- Eli Lilly & Company
Recent Developments
- In June 2024, the FDA approved AbbVie’s RINVOQ (upadacitinib) for children and adolescents living with active polyarticular juvenile idiopathic arthritis or juvenile psoriatic arthritis. The availability of a JAK inhibitor for these conditions further highlights the importance of early diagnostic workups—such as imaging and inflammation-related biomarkers to identify patients who may benefit from advanced therapies.
- Also in June 2024, Regeneron and Sanofi received FDA approval for KEVZARA (sarilumab) to treat active polyarticular juvenile idiopathic arthritis. Since KEVZARA targets the IL-6 pathway, its clinical use reinforces reliance on testing strategies that monitor inflammatory activity, including ESR and CRP evaluations, as well as imaging tools that track joint damage over time.
Report Scope
Report Features Description Market Value (2024) US$ 6.4 Billion Forecast Revenue (2034) US$ 9.6 Billion CAGR (2025-2034) 4.1% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Test (Blood Tests, ESR, Anti-Nuclear Antibody, C-Reactive Protein and Others), By End-user (Hospitals and Research Laboratories) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Johnson & Johnson Services, Inc., Novartis International AG, Bristol-Myers Squibb Company, Zydus Cadila, Genentech, Inc., Latona Life Sciences, AbbVie, Eli Lilly & Company Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Juvenile Idiopathic Arthritis Diagnostic MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample
Juvenile Idiopathic Arthritis Diagnostic MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Johnson & Johnson Services, Inc.
- Novartis International AG
- Bristol-Myers Squibb Company
- Zydus Cadila
- Genentech, Inc.
- Latona Life Sciences
- AbbVie
- Eli Lilly & Company










