Global Interventional Cardiology Devices Market, By Product (Angioplasty Balloons, Coronary Stents, Angioplasty Catheters, Endovascular Aneurysm Repair Stent Grafts, Other products), By End-User (Hospitals, Ambulatory Surgical Centers, Cardiac Catheterization Laboratories, and Other End-users), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 102745
- Number of Pages: 348
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
The Global Interventional Cardiology Devices Market size is expected to be worth around USD 38 Billion by 2032 from USD 18 Billion in 2022, growing at a CAGR of 8.0% during the forecast period from 2023 to 2032.
The large patient population, high mortality rate from coronary artery disease, and predicted rise in demand for cardiovascular devices all contribute to the market’s development. The treatment of structural heart problems with a catheter is the focus of interventional cardiology. A catheter-based technique falls within the category of peripheral vascular devices, and it is applied to the arms or legs. Due to a number of factors, including intense price competition, declining ASPs will restrain industry development.
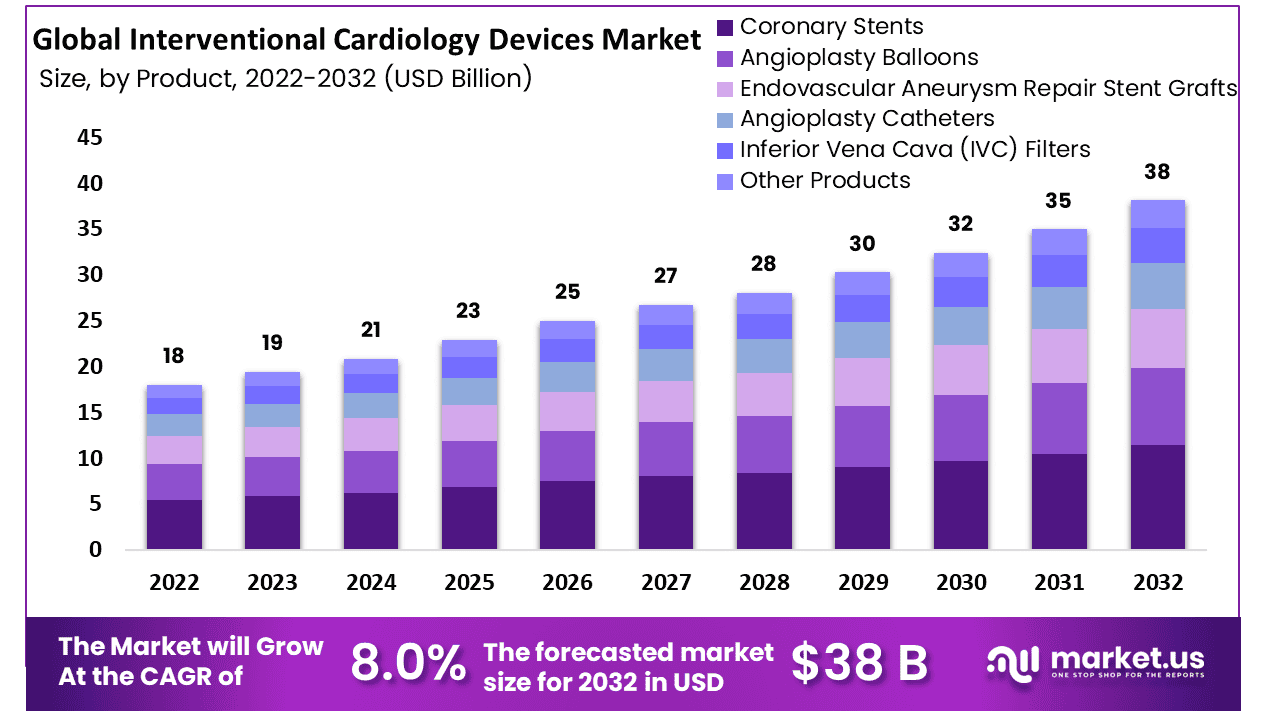
*Actual Numbers Might Vary In The Final Report
Key Takeaways
- Lifesaving Innovations: The Interventional Cardiology Devices Market is propelled by life-changing innovations. Interventional devices play an integral part in diagnosing and treating cardiovascular disorders with less-invasive surgical solutions that offer minimally invasive solutions than their predecessors.
- Diverse Devices: The market offers a diverse array of devices for diagnosing or treating heart conditions, from coronary stents and catheters, balloons and atherectomy devices to intravascular imaging systems and atherectomy devices. Each serves a distinct function in diagnosing or treating cardiovascular ailments.
- Increase in Cardiovascular Disease Burden: With cardiovascular diseases on the rise, interventional cardiology devices have become an essential asset to maintaining heart health and avoiding future attacks – saving lives along the way! They restore blood flow back into your heart while protecting from potential heart attack episodes – such as using devices designed specifically to restore it during heart attacks or prevent them altogether.
- Interventional cardiology procedures are known for being minimally invasive, cutting recovery times and hospital stays down significantly for both patients and healthcare providers alike. Therefore, they have become an increasingly popular option among both parties involved.
Product Analysis
Coronary stents had the highest revenue share more than 35.0%. The majority of sales are typically made by Drug-eluting stents (DES) in the coronary stent market as a whole. The market has benefited from the introduction of next-generation DES including Medtronic’s Resolute Onyx, Abbott Laboratories’ XIENCE series, and numerous other popular stents like the Orsiro DES and SYNERGY from BIOTRONIK and Boston Scientific. These more contemporary stents provide better stent integrity, deliverability, and fewer rates of issues than their more conventional counterparts.
The percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market will expand significantly. This is partial because there will be more percutaneous coronary intervention (PCI) procedures performed, particularly in places like Latin America, India, and China where untreated CAD is more common.
End-User Analysis
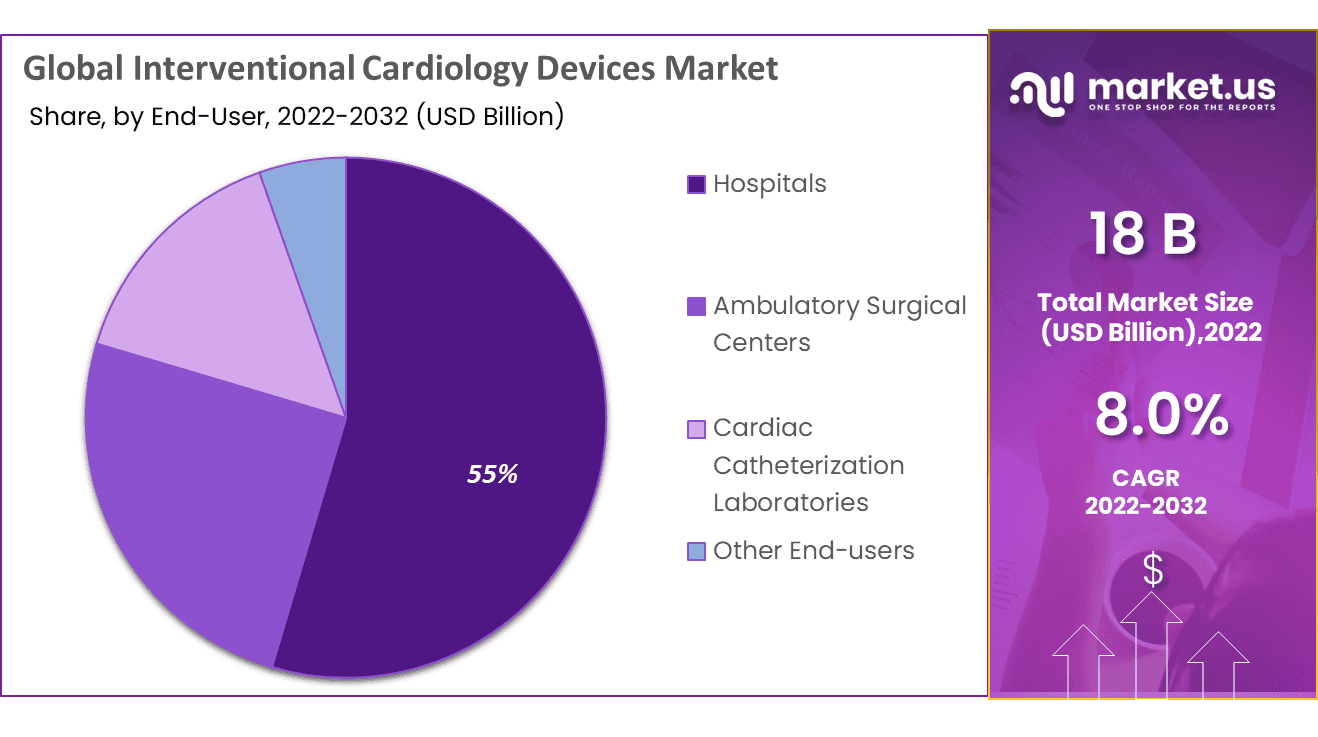
The Hospital sector dominates the market with the largest market share of 54.6%. Cardiology deals with heart-related diseases and problems. Hence, a medical specialist known as a “cardiologist” can identify and assist in the treatment of cardiac abnormalities and heart disorders such as heart failure, coronary artery disease, electrophysiology, etc.
Key Market Segments
Based on Product
- Angioplasty Balloons
- Coronary Stents
- Angioplasty Catheters
- Endovascular Aneurysm Repair Stent Grafts
- Inferior Vena Cava (IVC) Filters
- Plaque Modification Devices
- Hemodynamic Flow Alteration Devices
- Other Products
Based on End-User
- Hospitals
- Ambulatory Surgical Centers
- Cardiac Catheterization Laboratories
- Other End-users
Drivers
Increasing the use of tobacco products and the prevalence of obesity
Obesity and smoking are both closely related to cardiovascular illnesses. Plaque buildup in blood arteries is accelerated by smoking. Moreover, the chemicals in cigarette smoke, cause the blood to thicken and clot in veins and arteries. The arteries that supply blood to the heart become constricted by plaque or obstructed by clots when a person has coronary heart disease.
According to the CDC, smoking-related deaths affect more than 480,000 people only in the US each year. Every year, smoking cigarettes causes the deaths of more than 7 million people worldwide. Around 7 million people each year die as a result of cigarette smoking worldwide. More than 8 million individuals per year are anticipated to pass away from illnesses brought on by tobacco smoking by 2032.
In the same way that smoking causes heart disease. Blood pressure rises in obese people because they normally need more blood to provide oxygen and nourishment to their bodies. Heart disease is frequently caused by high blood pressure. Hence, it is anticipated that the demand for cardiovascular devices, especially interventional cardiology equipment, would rise in the coming years as a result of cigarette smoking and obesity.
Restraints
High Cost
Interventional cardiology devices and techniques can be expensive. The cost includes not only the devices themselves but also associated equipment, consumables, and hospital charges. For patients and healthcare systems with low resources, the high cost may be a barrier to access. In underdeveloped countries in particular, it may have an effect on the adoption of interventional cardiology equipment.
Alternative Treatment Options
The availability of alternative treatments, like medication-based therapy or surgical procedures, may affect the demand for interventional cardiology equipment. Alternative treatment options may be preferred over interventional procedures, depending on the condition of the patient, their preferences, and advice from healthcare professionals. This may limit the market for interventional cardiovascular devices.
Opportunity
Technological Developments
Interventional cardiology technologies, such as imaging modalities, catheter-based systems, and implantable devices, are constantly improving. These developments present possibilities for the development of unique and superior interventional cardiology devices. For instance, combining robotics, artificial intelligence (AI), and advanced imaging methods can improve the accuracy of procedures and patient outcomes.
Emerging Markets
Demand for interventional cardiac devices is growing in countries including Asia, Latin America, and the Middle East. These regions have experienced rapid economic growth and improvements in healthcare infrastructure. As healthcare access improves and awareness about interventional cardiology procedures grows, demand for devices like coronary stents and catheters will increase. This will create opportunities for the market to expand.
Trends
Increased Incidence of Cardiovascular Diseases: With the rising incidence of cardiovascular diseases such as coronary artery disease and peripheral vascular disease, there has been an increasing demand for interventional cardiology devices.
Technological Advancements: With the advancements in interventional cardiology, there has been an explosion of new devices with advanced features that offer greater precision, ease of use, and better patient outcomes. These innovations continue to shape the marketplace today.
Regional Analysis
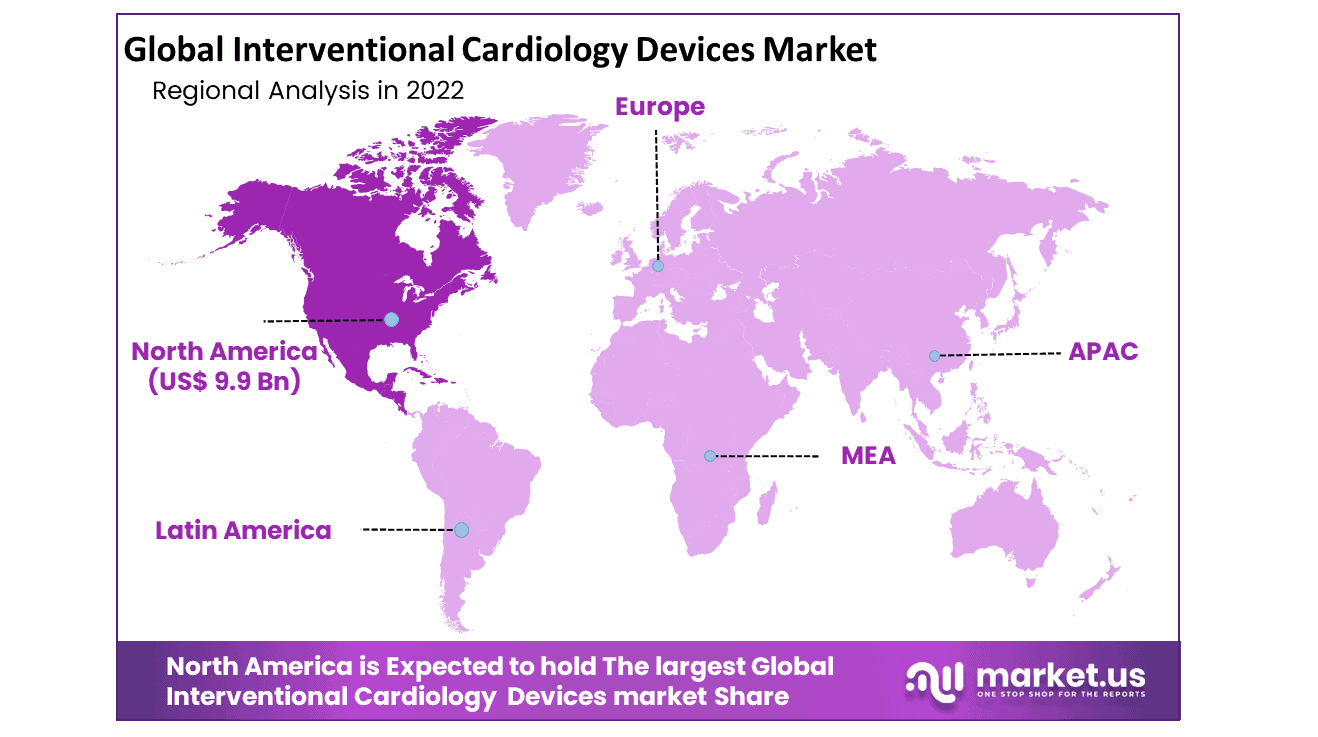
The market for interventional cardiology devices is dominated by North America. Because of the rising elderly and pediatric populations. The Asia-Pacific is the area with the second-fastest growth in the market for interventional cardiology devices. Asia-Pacific expansion is being driven by a rising awareness of the ease that interventional cardiology devices offer.
The market for interventional cardiology devices in APAC has also been expanding as a result of increased expenditures in the healthcare industry. Moreover, important main companies for aortic endograft surgery in the Asia-Pacific area include Cook Medical, Gore, and Medtronic. These elements support the expansion of the Asia-Pacific region in the market for interventional cardiology devices.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Interventional cardiology is a very competitive marketplace. Boston Scientific, Medtronic, and Abbott Laboratories are a few of the well-known market leaders in the IC devices industry. These businesses have developed into dominating players as a result of their broad product ranges and capacity to meet a number of market niches.
They are able to adopt aggressive pricing strategies since they could offer full product bundles of the IC devices needed in cardiac catheterization facilities for substantially less money. Also, these companies have been investing in the creation of fresh goods for the lucrative intravascular imaging industry and the coronary stent market.
Market Key Players
- Abbott Laboratories
- Medtronic
- Boston Scientific
- Spectranetics
- Cardinal Health
- Philips Healthcare
- Terumo
- Alvimedica
- Teleflex Medical
- Braun
- Biosensors International
- Meril Life Sciences
- Biotronik
- ACIST Medical Systems
- Asahi Intecc
- CID Vascular
- Cook Medical
- Medinol
- Merit Medical Systems
- Numed
- Opsens
- Vascular Solutions
- Zeon Medical
- Other Key Players
Recent Developments
- For the treatment of lengthy lesions in the coronary arteries, iVascular announced the introduction of additional lengths for the Angiolite drug-eluting stent (DES). The European CE Mark has approved the use of the 44- and 49-mm lengths for the treatment of diffuse lesions.
- Ergonomic surgical laparoscopic scissors have been released by Becton & Dickinson as part of their new Snowden-Pencer product line. Reusable handles and disposable tips from Snowden-Pencer deliver cutting-edge performance for the greatest cutting results. With their click-fit scissors tips, which offer aural and tactile feedback to ensure correct assembly, they are simpler to put together. A precise grip is made possible by Snowden-Pencer, speeding up surgical processes.
- The SAPIEN 3 Transcatheter Heart Valve was given Chinese regulatory clearance by Edward Lifesciences Company (US). This medication is used to treat individuals with severe, symptomatic aortic stenosis (AS), particularly those who cannot have open heart surgery.
Report Scope
Report Features Description Market Value (2022) USD 18 Bn Forecast Revenue (2032) USD 38 Bn CAGR (2023-2032) 8.0% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product, By End-User Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Abbott Laboratories, Medtronic, Boston Scientific, Spectranetics, Cardinal Health, Philips Healthcare, Terumo, Alvimedica, Teleflex Medical, B. Braun, Biosensors International, Meril Life Sciences, Biotronik, ACIST Medical Systems, Asahi Intecc, CID Vascular, Cook Medical, Medinol, Merit Medical Systems, Numed, Opsens, Vascular Solutions, Zeon Medical, Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the Interventional Cardiology Devices Market?The Interventional Cardiology Devices Market refers to the market for medical devices used in interventional cardiology procedures. These devices are used to diagnose and treat cardiovascular conditions through minimally invasive techniques.
What are some common types of Interventional Cardiology Devices?Common types of Interventional Cardiology Devices include coronary stents, catheters, guidewires, balloon angioplasty devices, atherectomy devices, and embolic protection devices.
What is the purpose of Interventional Cardiology Devices?Interventional Cardiology Devices are used to diagnose and treat various heart conditions, including coronary artery disease, heart valve disorders, congenital heart defects, and peripheral vascular diseases. These devices help restore blood flow, repair damaged blood vessels, and improve overall heart function.
How does the Interventional Cardiology Devices Market contribute to patient care?The Interventional Cardiology Devices Market provides healthcare professionals with advanced tools and technologies to perform minimally invasive procedures, reducing the need for traditional open-heart surgeries. This results in shorter hospital stays, faster recovery times, and improved patient outcomes.
 Interventional Cardiology Devices MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Interventional Cardiology Devices MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Abbott Laboratories
- Medtronic
- Boston Scientific
- Spectranetics
- Cardinal Health
- Philips Healthcare
- Terumo
- Alvimedica
- Teleflex Medical
- Braun
- Biosensors International
- Meril Life Sciences
- Biotronik
- ACIST Medical Systems
- Asahi Intecc
- CID Vascular
- Cook Medical
- Medinol
- Merit Medical Systems
- Numed
- Opsens
- Vascular Solutions
- Zeon Medical
- Other Key Players










