Global Achondrogenesis Market By TYPE (Type IA, Type IB, Type II) By DIAGNOSIS-(Physical examination, Molecular genetic testing(Chorionic villus sampling, Aminocentesis, Others) Biochemical testing) By END USER (Specialty Clinics, Hospitals, Diagnostic Centers, Other End-Users) By Region, and Key Companies-Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Jan 2024
- Report ID: 66553
- Number of Pages: 253
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
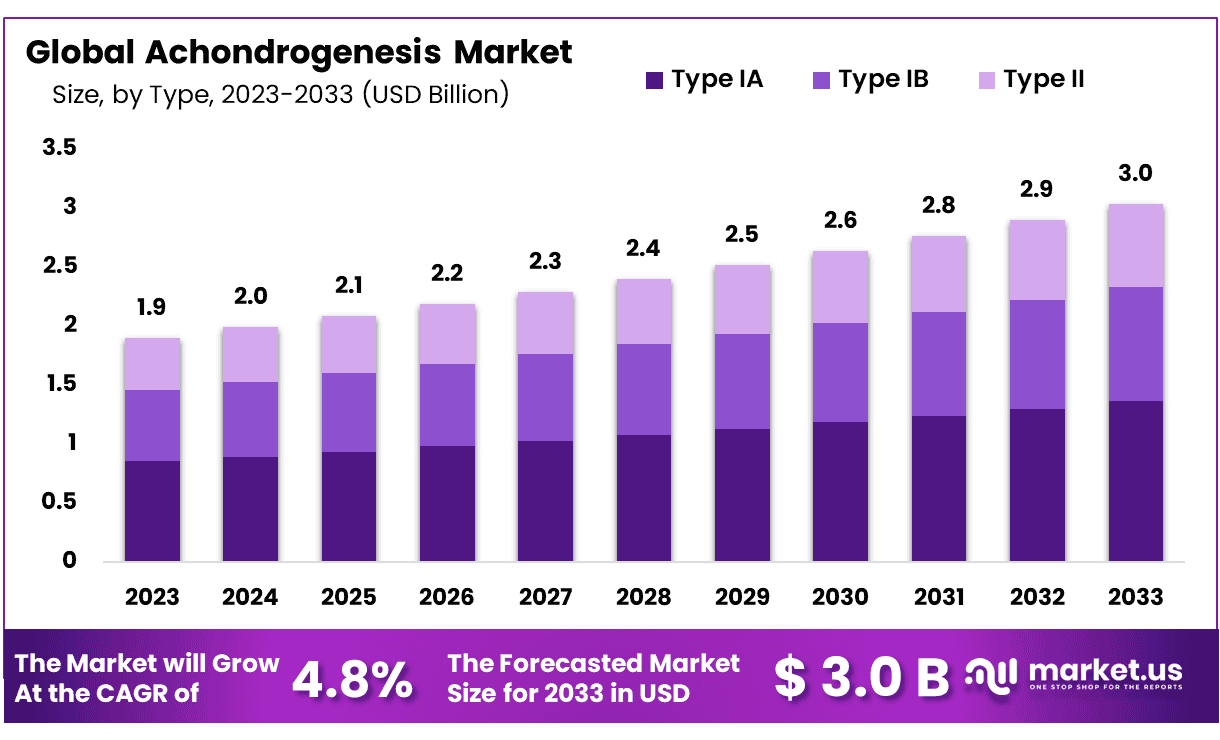
The Global Achondrogenesis Market size is expected to be worth around USD 3.0 Billion by 2033 from USD 1.9 Billion in 2023, growing at a CAGR of 4.8% during the forecast period from 2024 to 2033.
A highly uncommon genetic condition called aschondrogenesis alters how a person’s bones grow. It’s an inherited condition that exists from birth. Individuals who have achondrogenesis have severe issues with bone growth, which causes them to have very small chests, arms, and legs, as well as strange facial and head traits.
Because achondrogenesis is such a rare disorder, it’s crucial to realize that, in terms of market research, there might not be a significant market for medicines or therapies specifically for it. To better assist persons who have this illness and their families, medical professionals and researchers are currently working to gain a deeper knowledge of it. They concentrate on determining the cause, diagnosis, and potential treatments for individuals with achondrogenesis.
This report is all about the market in one or more industries. In particular, we’re looking at the Achondrogenesis Market. What’s interesting is that this report doesn’t just throw numbers at you; it gives you a mix of facts and insights. We’re looking ahead from 2024 to 2033 to see what might happen.
Key Takeaways
- Market Size: Achondrogenesis Market size is expected to be worth around USD 3.0 Billion by 2033 from USD 1.9 Billion in 2023.
- Market Growth: The market growing at a CAGR of 4.8% during the forecast period from 2024 to 2033.
- Type Analysis: Type IA, the most predominant form, commands a substantial 44.8% market share.
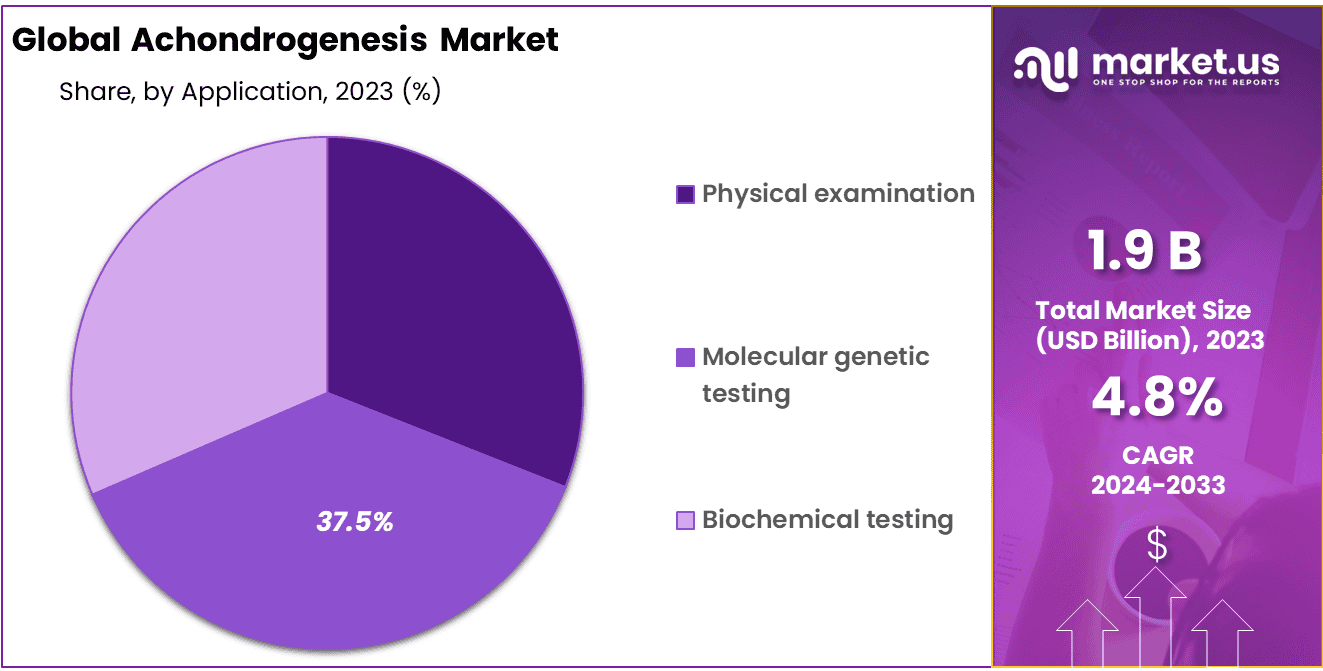
- Diagnosis Analysis: Molecular Genetic Testing takes the lead, holding a dominant 37.5% market share.
- End-Use Analysis: Hospitals dominate the achondrogenesis market with an estimated 44.5% share.
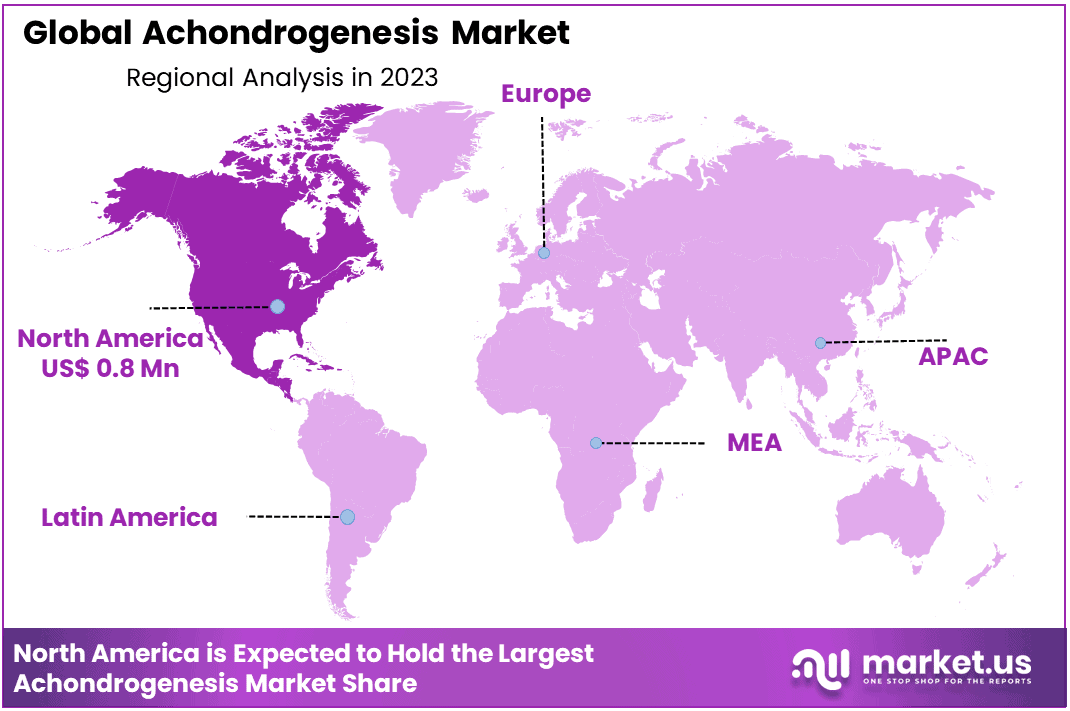
- Regional Analysis: North America dominant 43.2% market share and hold USD 0.8 million market revenue.
- Developments in Genetic Testing: The precision and early identification of Achondrogenesis have been facilitated by the increased diagnostic accuracy of this test due to technological advancements in genetic testing.
- Growing therapeutic developments: In the search for viable Achondrogenesis treatments, gene therapy and personalized medicine techniques are two growing developments.
- Restricted Patient Population: Research and development activities are impacted by the market’s difficulties stemming from the small number of people afflicted with achondrogenesis.
Type Analysis
In the realm of Achondrogenesis, a rare genetic disorder affecting skeletal development, market dynamics are notably influenced by the prevalence of different subtypes. Type IA, the most predominant form, commands a substantial 44.8% market share. This dominance is indicative of the higher occurrence of Type IA cases within the affected population.
Type IB and Type II Achondrogenesis, though less prevalent, remain significant players in this specialized market. Type IB, with its unique characteristics, caters to a distinct subset of patients, contributing to the diversity of available treatments and therapies. Meanwhile, Type II, while less prevalent than Type IA, underscores the need for comprehensive research and therapeutic options tailored to its specific clinical manifestations.
The Achondrogenesis market, driven by a commitment to understanding and addressing the needs of patients with these rare subtypes, exemplifies the importance of specialized research, tailored therapies, and a cautiously optimistic outlook toward advancing treatment options for those affected by this challenging condition. This data-driven approach ensures that resources are allocated efficiently, offering hope and improved quality of life for individuals battling Achondrogenesis.
Diagnosis Analysis
Diagnosis is characterized by several crucial methods, each playing a distinct role in identifying and understanding this rare genetic disorder. Among these methods, Molecular Genetic Testing takes the lead, holding a dominant 37.5% market share. This approach involves the examination of a patient’s DNA at the molecular level, allowing for precise detection and characterization of genetic mutations associated with Achondrogenesis.
Physical examination remains another vital diagnostic tool in this market. It offers clinicians the opportunity to observe and assess physical symptoms and skeletal abnormalities that are often indicative of Achondrogenesis. While not as prevalent as Molecular Genetic Testing, physical examination continues to be an essential component of the diagnostic process.
Biochemical testing, although representing a smaller market share, plays a valuable role in confirming and complementing diagnoses through the analysis of specific biomarkers and metabolic pathways associated with Achondrogenesis.
The Achondrogenesis market’s diagnostic landscape underscores the importance of a multi-faceted approach, where each method contributes to a comprehensive understanding of this rare condition, facilitating early intervention and improved patient outcomes. This data-driven, diversified approach is essential in the quest to advance the diagnosis and treatment of Achondrogenesis effectively.
End-user Analysis
Achondrogenesis is an extremely rare and fatal skeletal disorder characterized by severe, inadequate bone and cartilage formation. Given the rarity and severity of the condition, hospitals dominate the achondrogenesis market with an estimated 44.5% share. Hospitals have the necessary expertise and capabilities to accurately diagnose achondrogenesis through imaging tests and genetic analysis. They also provide essential supportive care to infants born with the condition, including respiratory assistance and pain management.
Specialty clinics account for around 31% of the achondrogenesis market. These dedicated centers have staff experienced in musculoskeletal disorders and provide genetic counseling as well as prenatal and postnatal care guidance for affected families. The remaining 24.5% share belongs to diagnostic centers which conduct tests like prenatal ultrasounds, x-rays, CT scans and DNA analysis to confirm achondrogenesis diagnoses. Overall, hospitals are the primary end-users in the achondrogenesis market today, but specialized clinics and diagnostics centers also play an important role in properly identifying cases and supporting patient care.
Key Market Segments
TYPE
- Type IA
- Type IB
- Type II
DIAGNOSIS
Physical examination
Molecular genetic testing
- Chorionic villus sampling
- Aminocentesis
- Others
Biochemical testing
END USER
- Specialty Clinics
- Hospitals
- Diagnostic Centers
- Other End-Users
Driver
Advancements in Genetic Testing
One of the primary drivers of the Achondrogenesis market is the remarkable progress in genetic testing technologies. Genetic testing plays a pivotal role in diagnosing and understanding rare genetic disorders like Achondrogenesis. With the development of high-throughput sequencing and next-generation sequencing techniques, clinicians can now identify the specific genetic mutations responsible for Achondrogenesis more accurately. This has led to early and precise diagnosis, enabling healthcare providers to offer tailored treatment and management options.
Increasing Awareness and Research Initiatives
The growing awareness about rare genetic disorders, including Achondrogenesis, has spurred research initiatives and collaborations among healthcare professionals, academic institutions, and pharmaceutical companies. These efforts have resulted in a deeper understanding of the disease’s pathophysiology, which, in turn, has paved the way for the development of potential therapeutic interventions. The rise in patient advocacy groups and support organizations has also played a significant role in driving research and raising funds for Achondrogenesis-related studies.
Trend
Gene Therapy and Personalized Medicine
A notable trend in the Achondrogenesis market is the emergence of gene therapy and personalized medicine approaches. Researchers are exploring innovative ways to correct or mitigate the genetic mutations responsible for Achondrogenesis. This trend aligns with the broader movement towards precision medicine, where treatments are tailored to an individual’s genetic makeup. While these therapies are in the experimental stage, they hold the promise of providing more targeted and effective solutions for Achondrogenesis patients in the future.
Regulatory Incentives for Rare Diseases
Another significant trend is the implementation of regulatory incentives to encourage the development of treatments for rare diseases, including Achondrogenesis. Governments and regulatory bodies in various countries are offering orphan drug status, expedited approval processes, and financial incentives to pharmaceutical companies engaged in rare disease research. This trend has stimulated interest in developing therapies for rare genetic disorders like Achondrogenesis, where the patient population is small but in need of specialized care.
Restraint
Limited Patient Pool
A key restraint in the Achondrogenesis market is the limited patient pool. Achondrogenesis is an extremely rare genetic disorder, and the number of affected individuals is relatively small. This poses challenges for pharmaceutical companies and researchers in terms of conducting clinical trials and achieving economies of scale. The small patient population can result in higher development costs per patient and a longer timeline for drug development.
Complexity of Genetic Interventions
The complexity of genetic interventions, such as gene therapy, presents a significant restraint. Developing and administering therapies that can accurately target and correct genetic mutations in individuals with Achondrogenesis is a technically demanding task. Safety concerns, ethical considerations, and long-term monitoring are crucial aspects that must be addressed. These challenges can slow down the progress of potential treatments for the disorder.
Opportunity
Investment in Rare Disease Research: An opportunity in the Achondrogenesis market lies in increased investment and funding for rare disease research. As governments, philanthropic organizations, and venture capitalists recognize the importance of addressing rare genetic disorders, there is a growing opportunity for researchers and pharmaceutical companies to secure financial support for their Achondrogenesis-focused projects. This influx of funding can accelerate research efforts and bring potential treatments to the forefront.
Collaboration and Knowledge Sharing
Collaboration among researchers, healthcare providers, and pharmaceutical companies presents an opportunity to pool expertise and resources for Achondrogenesis research. By sharing knowledge and data, the industry can collectively work towards a deeper understanding of the disorder and expedite the development of therapies. Collaborative efforts can also lead to more efficient clinical trial recruitment and a stronger collective voice in advocating for regulatory incentives.
Regional Analysis
In terms of geographical distribution, North America dominant 43.2% market share and hold USD 0.8 million market revenue, primarily driven by the heightened awareness and a surge in research and development initiatives. Europe, too, anticipates significant growth, driven by expanding distribution channels for product dissemination and continuous technological advancements. Meanwhile, the Asia-Pacific region is poised to secure the largest market share, attributable to a rising incidence of inherited disorders and growing government emphasis on the health of newborns.
Key Regions and Countries
North America
- The US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Player Analysis
The market report provides an in-depth analysis of the key players in the Achondrogenesis Market within the competitive landscape and company profile sections. These major players undergo comprehensive evaluation based on criteria including their product and/or service offerings, financial performance, noteworthy developments, strategic market approach, market position, geographic presence, and other significant attributes.
Additionally, this chapter offers a comprehensive insight into each player’s strengths, weaknesses, opportunities, and threats through a SWOT analysis. It also highlights the essential winning strategies, ongoing priorities, and the competitive challenges faced by the top three to five players in the market. Furthermore, it’s worth noting that the list of companies included in this market study can be customized to align with the specific requirements of the client.
Within the competitive landscape section of the report, readers can find detailed information about the top five companies, including their ranking, recent developments such as partnerships, mergers and acquisitions, and new product launches. The report also covers the geographical and industry footprint of these companies, as assessed through market analysis and the Ace matrix.
Market Key Players
- Thermo Fisher Scientific Inc.
- CooperSurgical, Inc.
- Illumina, Inc
- Siemens
- Bio-Rad Laboratories, Inc.
- FUJIFILM Holdings Corporation
- Koninklijke Philips N.V.
- Stryker
- TOSHIBA CORPORATION
- Invivoscribe, Inc.
- Abbott
- INVITROGEN CORPORATION
Recent Developments
- Thermo Fisher Scientific Inc.: Launched the Ion Torrent S5 Next-Generation Sequencing System in 2023, potentially enabling faster and more comprehensive genetic analysis for Achondrogenesis testing.
- CooperSurgical, Inc.: Expanded its portfolio of fetal monitoring and imaging technologies, which could be used for prenatal diagnosis of Achondrogenesis.
- Illumina, Inc.: Launched the NovaSeq 6000 Sequencing System in 2021, offering ultra-high-throughput sequencing for faster and more accurate Achondrogenesis testing.
- Siemens: Focused on developing advanced imaging technologies like MRI and CT scans for accurate diagnosis of Achondrogenesis at early stages.
- Bio-Rad Laboratories, Inc.: Developing new molecular diagnostic tools based on CRISPR technology for faster and more precise Achondrogenesis detection.
Report Scope
Report Features Description Market Value (2023) USD 1.9 Billion Forecast Revenue (2033) USD 3.0 Billion CAGR (2024-2033) 4.8% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By TYPE-(Type IA, Type IB, Type II);By DIAGNOSIS-(Physical examination, Molecular genetic testing(Chorionic villus sampling, Aminocentesis, Others) Biochemical testing);By END USER-(Specialty Clinics, Hospitals, Diagnostic Centers, Other End-Users) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Thermo Fisher Scientific Inc., CooperSurgical, Inc., Illumina, Inc, Siemens, Bio-Rad Laboratories, Inc., FUJIFILM Holdings Corporation, Koninklijke Philips N.V., Stryker, TOSHIBA CORPORATION, Invivoscribe, Inc., Abbott, INVITROGEN CORPORATION Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is Achondrogenesis?Achondrogenesis is an extremely rare genetic disorder characterized by severe skeletal abnormalities, leading to significant physical challenges.
How big is the Achondrogenesis Market?The global Achondrogenesis Market size was estimated at USD 1.9 Billion in 2023 and is expected to reach USD 3.0 Billion in 2033.
What is the Achondrogenesis Market growth?The global Achondrogenesis Market is expected to grow at a compound annual growth rate of 4.8%. From 2024 To 2033
Who are the key companies/players in the Achondrogenesis Market?Some of the key players in the Achondrogenesis Markets are Thermo Fisher Scientific Inc., CooperSurgical, Inc., Illumina, Inc, Siemens, Bio-Rad Laboratories, Inc., FUJIFILM Holdings Corporation, Koninklijke Philips N.V., Stryker, TOSHIBA CORPORATION, Invivoscribe, Inc., Abbott, INVITROGEN CORPORATION.
How is Achondrogenesis Diagnosed?Achondrogenesis is typically diagnosed through genetic testing, which identifies specific genetic mutations responsible for the disorder.
What are the Current Treatment Options for Achondrogenesis?There are currently no specific treatments for Achondrogenesis. Medical care primarily focuses on managing symptoms and providing supportive care.
What are the Key Challenges in the Achondrogenesis Market?One of the major challenges is the limited patient population, which can affect the feasibility of research and drug development efforts.

-
-
- Thermo Fisher Scientific Inc.
- CooperSurgical, Inc.
- Illumina, Inc
- Siemens
- Bio-Rad Laboratories, Inc.
- FUJIFILM Holdings Corporation
- Koninklijke Philips N.V.
- Stryker
- TOSHIBA CORPORATION
- Invivoscribe, Inc.
- Abbott
- INVITROGEN CORPORATION










