Global Preclinical CRO Market Size, Share, and Analysis By Services (Toxicology Testing, Safety Pharmacology, Drug Metabolism, Pharmacokinetics, IND Programs, and Others), by Model Type (Patient Derived Organoid (PDO) Model, Patient Derived Xenograft Model), By End User (Pharmaceutical and Biopharmaceutical companies, Medical Device manufacturing companies, and Academic Research Organizations, and Other End Users), and Regional Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 104628
- Number of Pages: 226
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
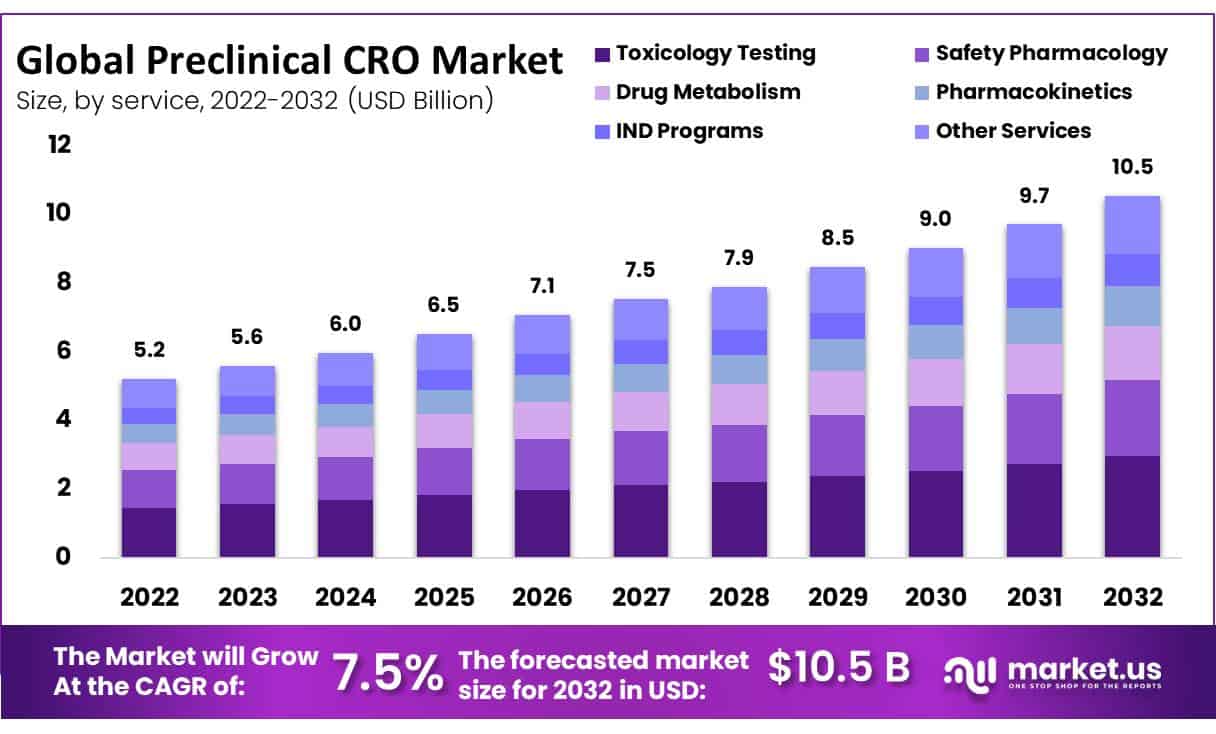
The Preclinical CRO Market size is expected to be worth around USD 10.5 Billion by 2032 from USD 5.2 Billion in 2022, growing at a CAGR of 7.5% during the forecast period from 2023 to 2032.
The market for preclinical research organization (CRO) is basically determined by an expansion in the volume of re-appropriating of different fundamental exercises, for example, clinical preliminary administrations, preclinical testing of medications and gadgets, and union of medications compounds, among others by the drug/biopharmaceutical as well as clinical gadgets organizations. The method involved with creating medications or clinical gadgets is basic and complex and requires colossal capital and assets for an ideal result. CROs lessen the organizations’ expenses for creating items in a specialty market.
In addition, they likewise offer direction from industry specialists while leading clinical preliminaries and help in improving the course of medications and gadgets’ entrance into the market. Hence, inferable from the different advantages CROs offer, the reception of CRO administrations has expanded fundamentally among new businesses with restricted assets.
Moreover, the preclinical CRO market is reinforced because of an expansion in the Research and development used by different medication and clinical gadget makers. The vast majority of the key clinical gadget organizations, drug, and biopharmaceutical fabricating organizations, at present, are vigorously putting resources into the research and improvement of novel and inventive items, which urges them to pick preclinical CRO administrations.
Key Takeaways
- Based on Services, toxicology testing generated substantial revenue in 2022, effectively making it the most contributing segment.
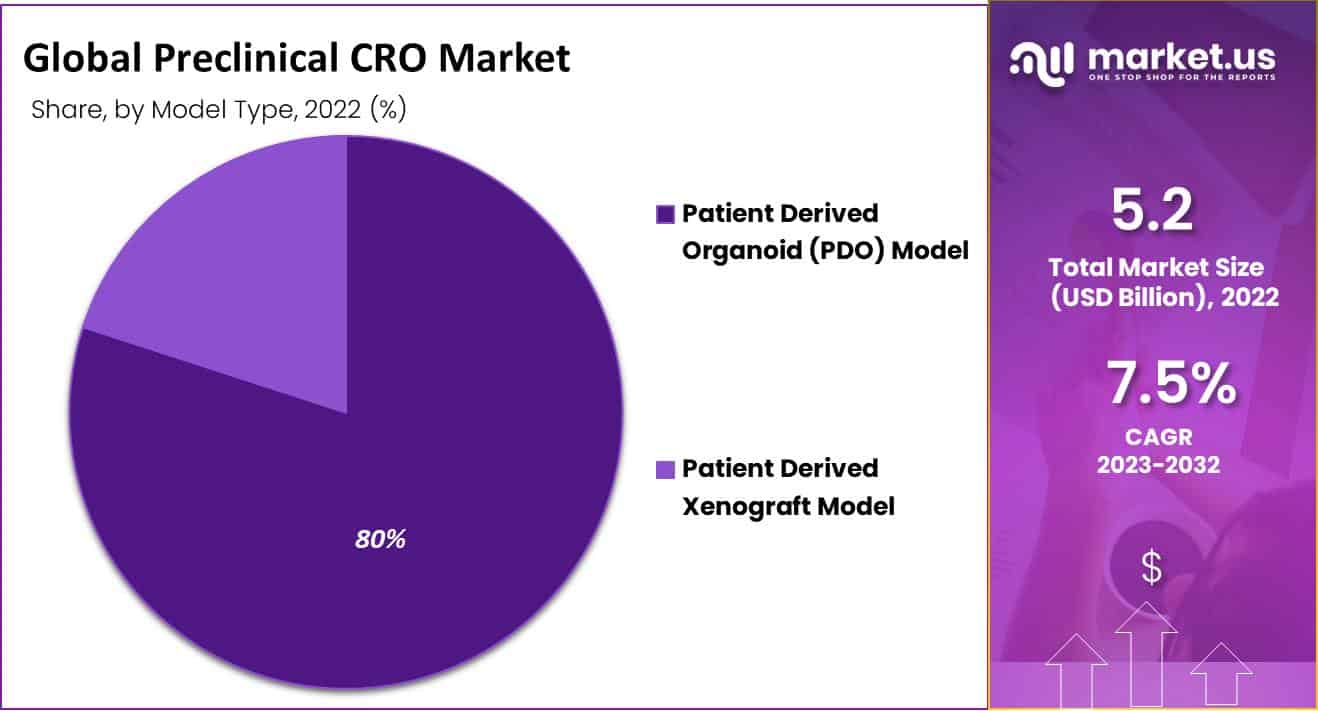
- When classified by Model Type, Patient-Determined Organoid (PDO) Model segment held the largest market share in the overall industry, adding up to 80%.
- As far as end user is considered, the Pharmaceutical and Biopharmaceutical Organizations segment held a major market share in 2022.
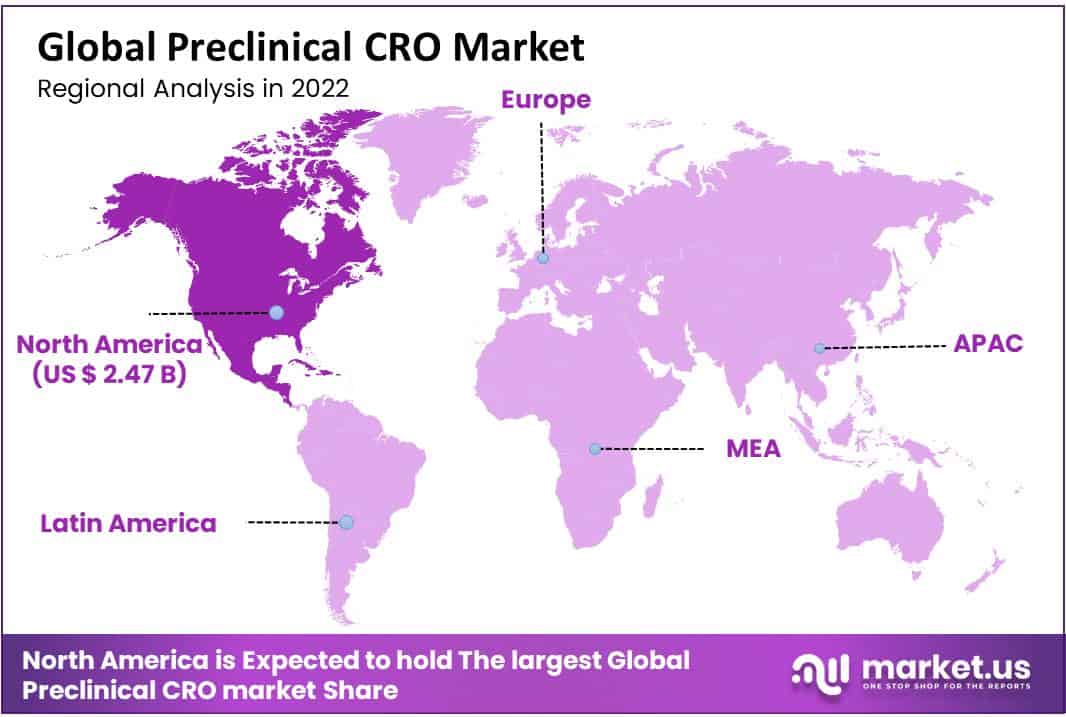
- North America maintained a stronghold on the market with a contribution of 47.50% in 2022.
- Asia Pacific is supposed to go through a time of remarkable development with a substantial CAGR as the forecast period progresses.
Services Analysis
Toxicology testing represents the biggest extent of the worldwide preclinical CRO market. Toxicology testing is fundamental and is central in the screening of novel and recently created drugs prior to testing them on people. Through this testing, the researchers not just test the well-being and viability of the medication yet, in addition, can describe the conceivable poisonous impacts it can create. Organizations’ dynamic in preclinical CRO administrations extending their administrations in toxicology testing is probably going to push the market.
The bioanalysis and DMPK concentrate on the section that is supposed to enlist the quickest CAGR of 8.5% during the gauge time frame. The portion is supposed to observe worthwhile development by virtue of an ascent in the interest for pharmacokinetic administrations to help toxicology tests for IND-empowering review. Furthermore, bioanalysis and DMPK studies are fundamental in the whole medication improvement process. They are acted in each phase of the medication improvement process and are not restricted to the preclinical stage. These variables are further adding to the portion development.
Model Type Insights
The Patient-Determined Organoid (PDO) Model segment held the largest share of 80.47% in 2022. The growing effect of the patient-inferred organoid (PDO) model is because of the utilization of direct gotten cells and tissues from the patient. This assists in customized healthcare, and the examples with canning be cryopreserved. For these reasons, they are turning into a fundamental piece of preclinical examinations as they help in speedier conclusion and guess of harm.
The Patient-determined xenograft model market has been dissected to develop consistently during the forecast time frame. This is credited to the growing number of CROs keeping an in-house stock of immune-deficient mice with patient-determined xenografts (PDXs). Moreover, this kind of examination permits researchers to co-relate laboratory research with people, attributable to the upkeep of the first hereditary cosmetics of the cancer cells. Likewise, the reactions seen in clinical trials among patients have been found to relate with the reactions in these patient-determined xenografts, which thusly considered a superior safety profile aDnd, in this manner, facilitates the endorsement of New Drug Examination (NDA).
End-User Analysis
The drug and biopharmaceutical organizations hold the biggest extent among the wide range of various end clients in the worldwide preclinical CRO market. The greater part of the drug and biopharmaceutical organizations are widely reevaluating their capabilities to limit costs.
The public authority and scholarly organizations fragment is assessed to enroll the quickest development of 8.2% during the figure time frame. The scholarly community and government bodies assume a critical part in the preclinical period of revelation and improvement. Moreover, a rising number of scholarly organizations and government bodies re-appropriating preclinical administrations to CROs will support fragment development. Scholarly establishments are one of the significant wellsprings of income for enormous CROs, like Charles Waterway Labs and LabCorp.
Key Market Segments
By Service
- Toxicology Testing
- Safety Pharmacology
- Drug Metabolism
- Pharmacokinetics
- IND Programs
- Other Services
By Model Type
- Patient Derived Organoid (PDO) Model
- Patient Derived Xenograft Model
By End-User
- Pharmaceutical and Biopharmaceutical companies
- Medical Device manufacturing companies
- Academic Research Organizations
- Other End Users
Drivers
Growth In Outsourcing Of Non-Core Function
A preclinical CRO (Contract Research Organization) is a helpful community that gives skills in research and improvement. It guarantees that a medicine or restorative contraption is protected and effectively created prior to being sent off on the lookout. It offers benefits that help with clearing a drug thing through creature testing and propelling it to the clinical stage.
Besides, it gives basic examinations to researchers, helpful labor force, various businesses, and authoritative contacts for evaluating drug adequacy and well-being in creature models and finishing Investigational New Medication (IND) recording studies.
The arising pattern of reevaluating addresses one of the key variables affecting market development. Aside from this, preclinical CROs offer start-to-finish administrations, for example, toxicology testing, which is additionally adding to the market development.
Moreover, the market has seen a huge change during the time spent on drug endorsement by the Food and Drug Administration (FDA). The interaction currently subjects the medication contender to preclinical investigations for laying out its security and effectiveness among people before the endorsement.
Other than this, the 21st Century Fixes Act was passed by the US Congress in 2016 to speed up clinical item improvement and attach the endorsement cycle for the send-off of clinical gadgets and medications. These drives are decidedly impacting the interest in preclinical CRO administrations.
Another central point, including the promptly accessible talented HR, the minimal expense of gadgets, and the rising pervasiveness of persistent infections, are expected to fuel the development of the market.
Restraints
Lack of skilled labor to hamper market growth
Due to the lack of skilled personnel in the CRA sector, market growth is likely to be hampered. As CRO is involved in R&D activities, it would be necessary for staff to possess a certain amount of technical expertise and refined skills. However, there is a shortage of skilled labour. In addition, the labour costs in the CRO market are substantially higher. This could pose a challenge to the growth of the market.
Opportunities
Possibility For Medical Device Businesses To Outsource Preclinical Studies
The global market has been driven in recent years by the predicted outsourcing of medical device testing to preclinical contract research organizations (CROs).
Impact of Macroeconomic Factors
Inflation has impacted economies worldwide, and has affected businesses operating on all strata. This particular macroeonomic factor has caused a notable increase in costs of living universally, which has rendered some economies incapable of attaining sustainable growth. Further, with rising price, production costs and labor costs have experienced an increase as well. This has discouraged businesses from initiating the process of product development, which has inevitably hampered the development of the preclinical CRO market.
Trends
Companies specialising in medical devices have increased their outsourcing of preclinical clinical research. Preclinical CROs are cost effective and efficient in a variety of ways. A pharmaceutical company can effectively conduct research with a faster turnaround by using the services of a Contract Research Organization. This has led to a further growth of the market.
Regional Analysis
North America To Exert Dominance In Global Market
North America represents the largest share of 47.50% in 2022, attributable to the presence of laid-out CROs having some expertise in earlier drug discovery, like Charles Waterway Research facilities and LabCorp. The U.S. is the greatest marketplace for preclinical trial outsourcing, as a few biopharmaceutical companies favor re-appropriating their preclinical trials to CROs situated in the U.S. to look for help from the Investigational New Drug (IND) application approved by the Food and Drug Administration.
Asia Pacific is likewise expected to develop at the fastest pace of 10.9% during the forecast time frame. The changing plan of action of MNC re-appropriating and the increasing expense of Research and development is supposed to increment preclinical re-appropriating in the Asia Pacific, attributable to the expense proficiency presented by CROs in nations like India and China. Laid-out companies situated in Western Europe and the U.S. seek logical administrations, site research advancement, and clinical exercises in the Asia Pacific area to lessen the expense related to research.
Key Regions
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The market players in the Preclinical CRO industry are serious and competitive in completing techniques for dealing with their portion on the lookout. Various systems embraced by the players are acquisitions, new fundamental farewells, and facilitated endeavors, among others. For instance, in December 2021, Labcorp procured Toxikon Corporation, which is a contract research organization. This obtaining will assist Labcorp with the development and extension of CRO administrations in pharmaceuticals and medical devices.
Essentially, in August 2021, Eurofins Scientific cooperated with Combination Antibodies; the organization is to give the best antibodies to many sicknesses. The understanding for this association is for two years, and in this, trend-setting innovation will be utilized. A few conspicuous players in the worldwide Preclinical CRO market include. PAREXEL International Corporation, Laboratory Corporation of America Holdings, Medpace, Inc., Envigo Corporation, Charles River Labs, PRA Health Science, Inc., PPD Inc., Covance Inc.
Market Key Players
With the presence of many local and regional players, the market for preclinical CRO is fragmented. Market players are subject to intense competition from top market players, particularly those with strong brand recognition and high distribution networks. To stay on top of the market, companies have gained various expansion strategies such as partnerships and product launches.
The following are some of the major players in the Global Preclinical CRO industry
- PAREXEL International Corporation
- Laboratory Corporation of America Holdings
- Medpace, Inc.
- Envigo Corporation
- Charles River Labs
- PRA Health Science, Inc.
- PPD Inc.
- Covance Inc.
- Other Key Players.
Recent Developments
- July 2023: the purchase of Enzo Biochem’s assets was completed in accordance with the Asset Purchase Agreement between Enzo and Labcorp. Enzo’s clinical labs were sold to Labcorp for over $113 million.
- July 2023: TransCure bioServices, a enetered into a collaboration with Preclina Inc. , a preclinical CRO based in South Korea. This partnership will enable Transcure to enter the Asia Pacific market.
Report Scope
Report Features Description Market Value (2022) USD 5.2 Bn Forecast Revenue (2032) USD 10.5 Bn CAGR (2023-2032) 7.5% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Service – Toxicology Testing, Safety Pharmacology, Drug, Metabolism, Pharmacokinetics, IND Programs, Other Services
By Model Type – Patient Derived Organoid (PDO) Model, Patient Derived Xenograft Model
By End-User – Pharmaceutical and Biopharmaceutical companies, Medical Device manufacturing companies, Academic Research Organizations, and Other End UsersRegional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA. Competitive Landscape Eurofins, PAREXEL International Corporation, Laboratory Corporation of America Holdings, Medpace, Inc., Envigo Corporation, Charles River Labs, PRA Health Science, Inc., PPD Inc., Covance Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three license to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What Is a Preclinical CRO?A Preclinical Contract Research Organization (CRO) is a company that conducts research and testing on pharmaceuticals and medical devices before they enter clinical trials.
How big is the Preclinical CRO Market?The global Preclinical CRO Market size was estimated at USD 5.2 billion in 2022 and is expected to reach USD 10.5 billion in 2032.
What is the Preclinical CRO Market growth?The global Preclinical CRO Market is expected to grow at a compound annual growth rate of 7.5 %. From 2023 To 2032
Who are the key companies/players in the Preclinical CRO Market?Some of the key players in the Preclinical CRO Markets are PAREXEL International Corporation, Laboratory Corporation of America Holdings, Medpace, Inc., Envigo Corporation, Charles River Labs, PRA Health Science, Inc., PPD Inc., Covance Inc., Other Key Players.
Why Are Preclinical CROs Important?Preclinical CROs play a crucial role in drug development by assessing the safety and efficacy of potential therapies, helping to bring safer and more effective medicines to market.
Who Uses Preclinical CRO Services?Pharmaceutical and biotechnology companies, academic institutions, and government agencies often utilize Preclinical CRO services for research and development.
What Types of Studies Do Preclinical CROs Conduct?These organizations perform a wide range of studies, including toxicity testing, pharmacokinetics, efficacy testing, and formulation development.
How Do Preclinical CROs Impact Drug Development?They accelerate drug development by providing specialized expertise, equipment, and resources, saving time and costs for pharmaceutical companies.

-
-
- PAREXEL International Corporation
- Laboratory Corporation of America Holdings
- Medpace, Inc.
- Envigo Corporation
- Charles River Labs
- PRA Health Science, Inc.
- PPD Inc.
- Covance Inc.
- Other Key Players.









