Global Patient Identification Wristbands Market By Product Type (Alert Wristband, RFID Wristband, Laser Wristband, Thermal Wristband and Others), By Material Type (Vinyl, Tyvek, Synthetic, Trilaminate and Non-tear Paper), By End-User (Hospitals (inpatient care), Specialty Clinics, Ambulatory Surgical Centers (outpatient surgery), Long-term Care Centers, Rehabilitation Centers, and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Dec 2025
- Report ID: 169656
- Number of Pages: 239
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
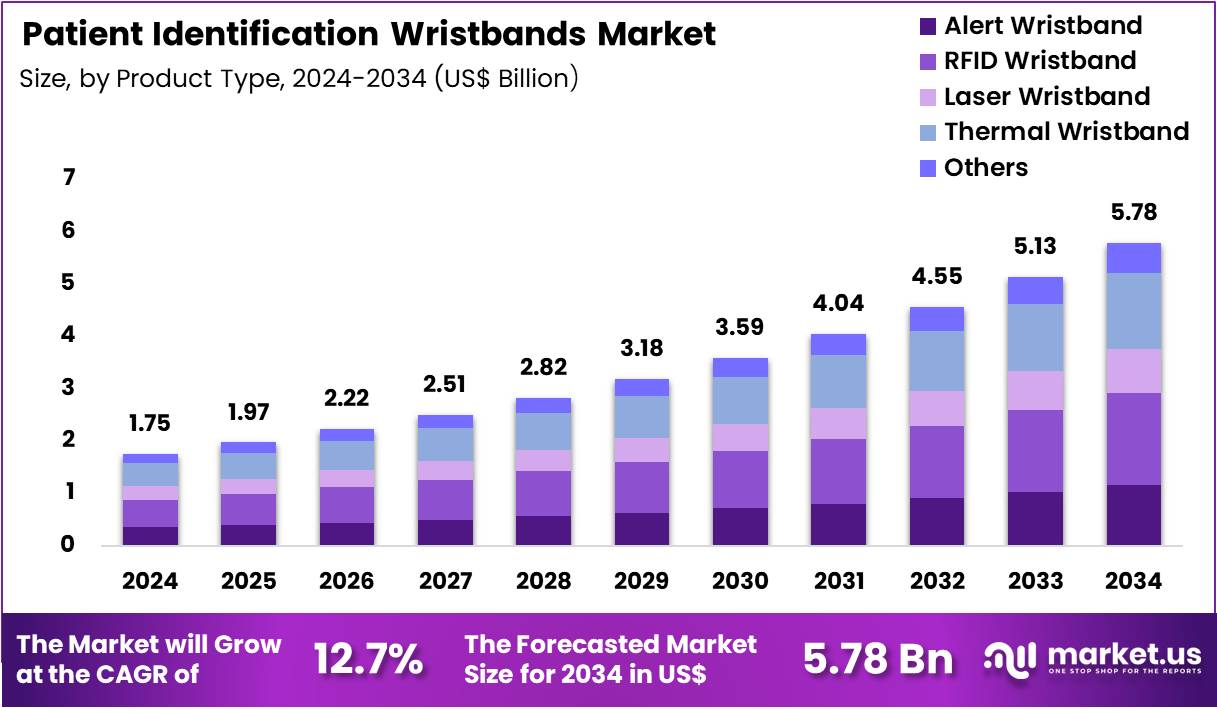
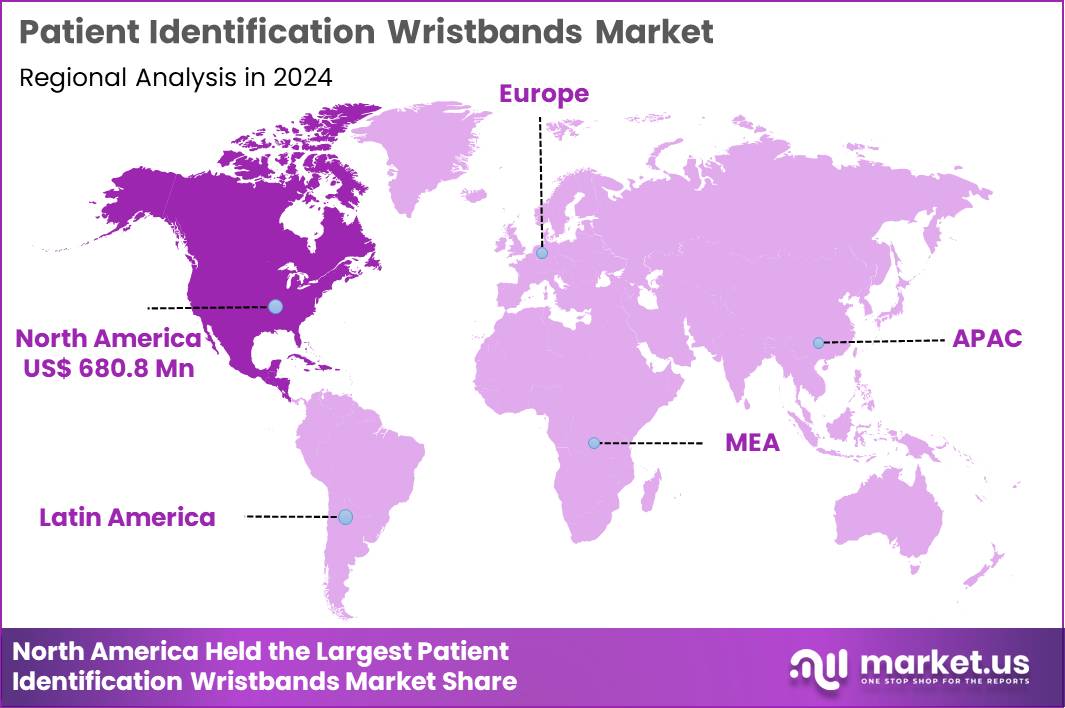
The Global Patient Identification Wristbands Market size is expected to be worth around US$ 5.78 Billion by 2034 from US$ 1.75 Billion in 2024, growing at a CAGR of 12.7% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 38.9% share with a revenue of US$ 680.8 Million.
The global patient identification wristbands market supports patient safety in healthcare settings by providing wearable bands for accurate identification during treatments, using materials like plastic, Tyvek, vinyl, and advanced options such as barcode or RFID-enabled versions.
The Patient Identification Wristbands Market represents a foundational component of global patient safety systems, supporting accurate identification across hospitals, clinics, rehabilitation facilities, surgical departments, and emergency care settings. Wristbands ensure correct matching of patients to medications, laboratory samples, surgical procedures, imaging reports, and clinical records, significantly reducing preventable medical errors. Healthcare authorities such as the World Health Organization and national safety agencies emphasize wristband-based verification as a mandatory patient-safety step, reinforcing their adoption, especially in high-acuity clinical environments.
Demand for wristbands continues to expand as hospitals transition from manual documentation to integrated digital workflows. The growing use of barcode and RFID systems for medication administration, blood transfusion verification, specimen tracking, and electronic health record (EHR) integration drives product innovation. For example, RFID wristbands increasingly support contactless scanning during infection outbreaks, helping minimize staff exposure and reduce workflow bottlenecks.
Moreover, advancements in printing technology, such as high-resolution thermal and laser printing, enhance durability, smudge resistance, and readability in critical care areas. The market benefits from rising surgical procedure volumes, higher inpatient admissions, and expanding neonatal and pediatric care programs.
Key Takeaways
- In 2024, the market generated a revenue of US$ 1.75 Billion, with a CAGR of 12.7%, and is expected to reach US$ 5.78 Billion by the year 2034.
- The Product Type segment is divided into Alert Wristband, RFID Wristband, Laser Wristband, Thermal Wristband, and Others, with RFID Wristband taking the lead in 2024 with a market share of 30.5%
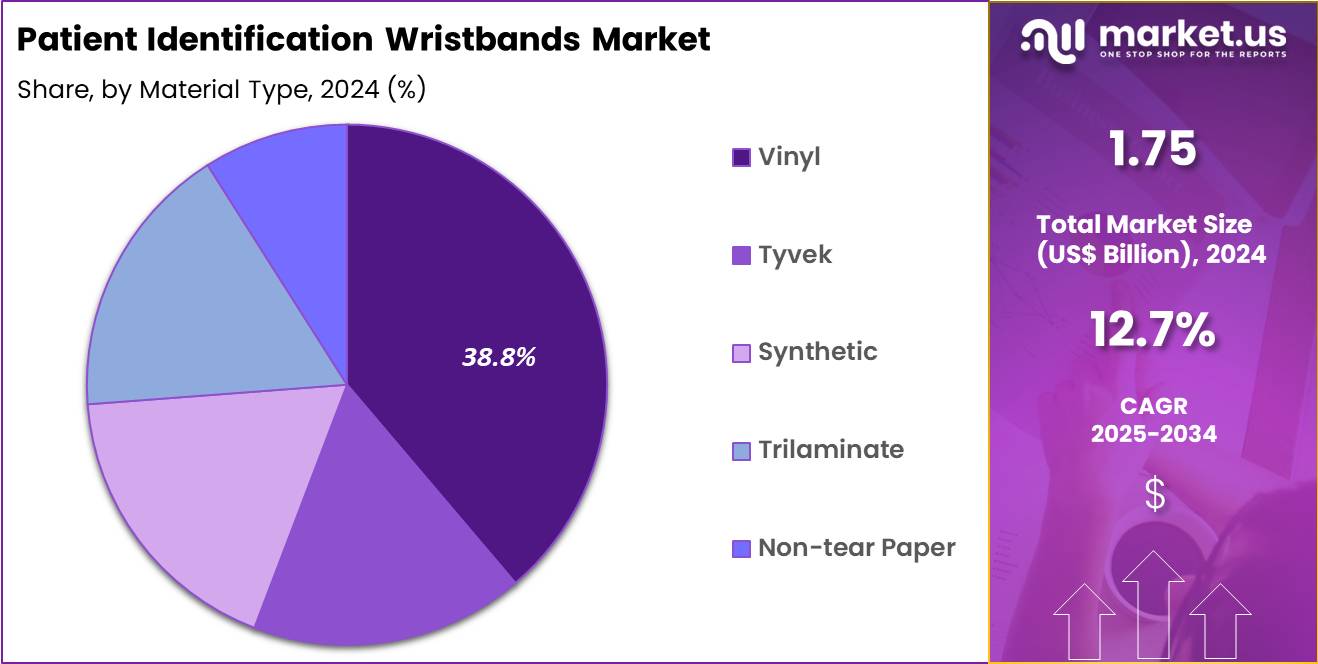
- The Material Type segment is divided into Vinyl, Tyvek, Synthetic, Trilaminate, and Non-tear Paper, with Vinyl taking the lead in 2024 with a market share of 38.8%
- The End-User segment is divided into Hospitals (inpatient care), Specialty Clinics, Ambulatory Surgical Centers (outpatient surgery), Long-term Care Centers, Rehabilitation Centers, and Others, with Hospitals taking the lead in 2024 with a market share of 58.9%
- North America led the market by securing a market share of 38.9% in 2024.
Product Type Analysis
RFID wristbands are the dominating category, supported by digital transformation in healthcare accounting for 30.5% market share in 2024. These wristbands enable automated patient identification, medication validation, and real-time location tracking. During infectious disease outbreaks, RFID supports contactless scanning, reducing exposure risk for clinicians. Pediatric hospitals use RFID tags to monitor infant movement, helping reduce abduction risks. Studies have shown that RFID-enabled medication verification can reduce administration errors by more than half, prompting broader adoption in intensive care and oncology units.
Alert wristbands hold a prominent share because global patient-safety guidelines require visible, color-coded identifiers for allergies, fall risks, restricted diets, and special medical conditions. A study by the Agency for Healthcare Research and Quality noted that allergy-related errors contribute significantly to avoidable adverse drug events, reinforcing the need for standardized alert wristbands.
Many hospitals use red wristbands for allergies, yellow for fall risk, and purple for do-not-resuscitate orders. Their adoption remains highest in emergency, anesthesia, and surgical departments where rapid visual identification is crucial. Laser wristbands remain widely used due to their compatibility with standard hospital printing systems and ease of integration with electronic health records.
They allow high-resolution printing of barcodes, patient demographics, and clinical instructions, ensuring accurate scanning across medication carts, laboratory analyzers, imaging devices, and pharmacy systems. Laser wristbands are particularly preferred in environments with high documentation loads, such as oncology day-care centers and pre-operative units.
Material Type Analysis
Vinyl is the leading material type which held majority market share of 38.8% in 2024 because it is durable, waterproof, and suitable for extended inpatient stays. It remains intact during showering, physiotherapy, and high-moisture environments. Neonatal units and ICUs prefer vinyl due to its ability to withstand repeated handling and device attachments. Vinyl bands also support multi-color coding systems and barcode compatibility.
Tyvek wristbands are lightweight and tear-resistant, making them ideal for short-stay patients, outpatient procedures, and emergency rooms. Their comfort and breathability benefit pediatric and geriatric populations. Tyvek is widely used during mass-casualty drills, vaccination drives, and disaster-response events due to quick application and durability. Synthetic wristbands offer flexibility, superior print quality, and high resistance to alcohol rubs used in clinical hygiene protocols. They are frequently used in infection-control units and oncology wards where frequent sanitization occurs.
Synthetic materials also support high-resolution printing for multi-barcode integration. Trilaminate wristbands provide extra strength, making them useful for psychiatric facilities, behavioral-health units, and high-movement patient groups. Their layered structure ensures that barcodes remain readable despite abrasion, making them essential for orthopedic rehabilitation and trauma-care centers.
End-User Analysis
Hospitals represent the largest end-user segment with 58.9% market share due to the scale of admissions across emergency, maternity, surgery, oncology, intensive care, and pediatric wards. Patient wristbands are mandatory in most inpatient environments, reinforcing compliance with global patient-safety goals. Wristbands ensure accurate specimen labeling, reduce medication errors, and support closed-loop blood transfusion systems. Increased surgical volumes and longer inpatient stays further strengthen demand.
Specialty clinics, such as cardiology, oncology, fertility, and dermatology centers, use wristbands primarily for day-care procedures, ambulatory infusions, minor surgeries, and diagnostic workflows. The rise of outpatient chemotherapy and chronic-care management programs increases the frequency of wristband use. Ambulatory surgical centers use wristbands extensively for patient verification before anesthesia, surgical incision, and post-operative monitoring.
Global growth in minimally invasive procedures fuels demand for wristbands that support high-accuracy barcode scanning throughout pre-operative, intra-operative, and recovery workflows. Long-term care facilities use wristbands for dementia care, chronic disease management, fall-risk monitoring, and resident tracking. RFID wristbands are increasingly used in memory-care units to prevent wandering and unauthorized exits, improving resident safety.
Key Market Segments
By Product Type
- Alert Wristband
- RFID Wristband
- Laser Wristband
- Thermal Wristband
- Others
By Material Type
- Vinyl
- Tyvek
- Synthetic
- Trilaminate
- Non-tear Paper
By End-User
- Hospitals (inpatient care)
- Specialty Clinics
- Ambulatory Surgical Centers (outpatient surgery)
- Long-term Care Centers
- Rehabilitation Centers
- Others
Drivers
Growing emphasis on reducing medical errors
A major driver in the Patient Identification Wristbands Market is the global emphasis on reducing medical errors, which remain a leading cause of preventable harm. Studies have shown that misidentification can contribute to medication errors, transfusion mismatches, and surgical mistakes. Wristbands provide a standardized, reliable method of confirming patient identity at every point of care.
Increasing adoption of barcode medication administration systems and digital EHR integration further accelerates wristband demand. For example, barcode scanning has been shown to reduce medication administration errors by more than 40 percent, reinforcing the requirement for high-quality wristbands. Growth in surgical procedures, emergency admissions, neonatal care programs, and chronic disease management also expands daily wristband usage across healthcare systems.
Restraints
Challenge of wristband durability and readability in real-world environments
A key restraint in the market is the challenge of wristband durability and readability in real-world environments. Poor-quality printing, moisture exposure, and material degradation can compromise barcode scanning accuracy. Allergic reactions to certain wristband materials also pose limitations, particularly in pediatric and dermatology wards.
Operational errors, such as incorrect printing or wristbands placed on the wrong patient, remain risks in busy clinical environments. Workflow interruptions occur when wristbands detach, smudge, or lose print clarity, creating delays in medication administration or testing. Limited awareness in smaller clinics and cost pressures in low-resource regions further restrict adoption of advanced wristband technologies.
Opportunities
Expansion of RFID-enabled patient identification
There is significant opportunity in the expansion of RFID-enabled patient identification, which supports contactless scanning, automated tracking, and real-time location systems. Growing investment in smart hospitals and digital health infrastructure fuels demand for intelligent wristbands that integrate with IoT platforms. Enhanced antimicrobial wristbands offer new opportunities in infection-control units.
Rising neonatal and maternity care volumes create opportunities for dual-identification wristbands that prevent mix-ups. Community health programs, vaccination campaigns, and mobile medical units also represent high-volume opportunities for low-cost wristband solutions. Increasing global focus on disaster preparedness and emergency response creates additional demand for durable, color-coded identification wristbands.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic conditions influence procurement patterns of healthcare facilities. During economic downturns, hospitals prioritize essential patient-safety tools such as wristbands, while postponing other non-critical investments. Fluctuations in raw material prices for vinyl, synthetic polymers, and printing supplies affect production costs and supply availability.
Geopolitical tensions can create disruptions in global trade routes, affecting the availability of specialized printing materials, RFID chips, and adhesive components. Public health emergencies, such as pandemics, drastically increase the consumption of wristbands due to surges in patient admissions, mass-screening events, and high patient turnover. Healthcare labor shortages also push hospitals toward automated workflows, increasing demand for RFID and barcode-integrated wristbands.
Latest Trends
Transition towards fully digital identification ecosystems
A key trend in the market is the transition toward fully digital identification ecosystems. Hospitals are integrating wristbands with EHR systems, enabling closed-loop verification for medications, transfusions, and laboratory specimens. RFID and NFC technologies are gaining traction in maternity wards, neonatal ICUs, and behavioral-health units. Antimicrobial wristbands are increasingly preferred in infection-prone environments such as ICUs.
Wristband personalization through QR codes and multilingual printing supports diverse patient populations. Another major trend is the adoption of environmentally friendly wristband materials as healthcare institutions pursue sustainability goals. Color-coded standardization across regions is also becoming more widespread, especially in emergency departments that rely on rapid visual identification.
Regional Analysis
North America is leading the Patient Identification Wristbands Market
North America represents the largest regional market due to its advanced healthcare infrastructure, high surgical volumes, and strong adoption of patient-safety protocols. The region enforces strict compliance with WHO and Joint Commission guidelines requiring patient identification at multiple care points. Hospitals in the United States and Canada routinely use barcode medication administration and electronic verification systems, increasing consumption of durable wristbands compatible with automated scanning.
Emergency departments and trauma centers across the region rely heavily on rapid wristband labeling during triage. The presence of leading wristband and identification system manufacturers further strengthens regional leadership.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is experiencing rapid growth due to expanding hospital capacity, rising surgical procedures, and increasing adoption of digital healthcare solutions. Countries such as China, India, Japan, and South Korea are modernizing their healthcare systems and integrating barcode and RFID-based patient verification. Large population sizes and increasing chronic disease incidence drive high patient throughput, boosting wristband usage.
Public health programs, vaccination campaigns, and emergency preparedness drills across Southeast Asia significantly increase demand for cost-efficient wristbands. Growing investment in maternity and neonatal care also supports adoption of specialized identification wristbands.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the market include Zebra Technologies Corporation, Precision Dynamics Corporation PDC Healthcare, Medline Industries LP, Thermo Fisher Scientific Inc., Endur ID Inc., IdentiSys Inc., Typenex Medical LLC, TIDI Products LLC, Avery Dennison Corporation, Brother Industries Ltd., Toshiba Tec Corporation, and Others
These companies shape the market through innovation in barcode and RFID technology, durable materials, and improved printing compatibility. Zebra Technologies and PDC Healthcare are widely adopted across hospitals for high-quality barcode printing and color-coded identification. Medline and TIDI Products supply large hospital networks with vinyl, synthetic, and Tyvek wristbands designed for extended wear.
RFID-focused firms such as Invengo and Identisys support digital verification and tracking systems across specialty hospitals and neonatal care units. The competitive landscape continues to shift toward smart wristbands with integrated sensors, antimicrobial coatings, improved comfort, and environmentally friendly materials.
Top Key Players
- Zebra Technologies Corporation
- Precision Dynamics Corporation PDC Healthcare
- Medline Industries LP
- Thermo Fisher Scientific Inc.
- Endur ID Inc.
- IdentiSys Inc.
- Typenex Medical LLC
- TIDI Products LLC
- Avery Dennison Corporation
- Brother Industries Ltd.
- Toshiba Tec Corporation
- Others
Recent Developments
- In March 2025, Zebra Technologies Corporation, announced that it will unveil new healthcare-focused innovations aimed at enhancing patient experience at the HIMSS25 Global Health Conference & Exhibition. The company will present these solutions at Booth 4043 from March 3 to 6, 2025, in Las Vegas, Nevada, where it will also demonstrate its RFID XR Wristband technology.
- In February 2023, HID, a global leader in trusted identity solutions, announced the acquisition of GuardRFID, a prominent provider of real-time location services (RTLS) hardware and software for the healthcare sector. This acquisition strengthens HID’s position in active RFID and RTLS technologies and broadens its ability to support emerging healthcare-focused use cases tailored to the operational needs of medical facilities.
- In November 2021, SATO launched a UHF RFID patient-ID wristband that uses a “super-soft antimicrobial material” designed for patient comfort and hygiene during hospital stays. The wristband supports encoding and printing on-demand (via SATO’s AEP printing platform), enabling hospitals to both print and program wristbands (e.g. barcode → RFID conversion) as needed.
Report Scope
Report Features Description Market Value (2024) US$ 1.75 Billion Forecast Revenue (2034) US$ 5.78 Billion CAGR (2025-2034) 12.7% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Alert Wristband, RFID Wristband, Laser Wristband, Thermal Wristband and Others), By Material Type (Vinyl, Tyvek, Synthetic, Trilaminate and Non-tear Paper), By End-User (Hospitals (inpatient care), Specialty Clinics, Ambulatory Surgical Centers (outpatient surgery), Long-term Care Centers, Rehabilitation Centers, and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Zebra Technologies Corporation, Precision Dynamics Corporation PDC Healthcare, Medline Industries LP, Thermo Fisher Scientific Inc., Endur ID Inc., IdentiSys Inc., Typenex Medical LLC, TIDI Products LLC, Avery Dennison Corporation, Brother Industries Ltd., Toshiba Tec Corporation, and Others Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Patient Identification Wristbands MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample
Patient Identification Wristbands MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Zebra Technologies Corporation
- Precision Dynamics Corporation PDC Healthcare
- Medline Industries LP
- Thermo Fisher Scientific Inc.
- Endur ID Inc.
- IdentiSys Inc.
- Typenex Medical LLC
- TIDI Products LLC
- Avery Dennison Corporation
- Brother Industries Ltd.
- Toshiba Tec Corporation
- Others










