Global Paper Diagnostics Market By Kit Type (Lateral Flow Assays, Paper Based Microfluidics, Dipsticks) By Device Type-(Diagnostic Devices, Monitoring Devices) By Application-(Clinical Diagnostics(Cancer, Liver Disorders, Infectious Diseases, Others) Environmental Monitoring, Food Quality Testing) By End-Use-(Hospital and Clinics, Homecare Settings, Other End-Uses) By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: July 2024
- Report ID: 52149
- Number of Pages: 248
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
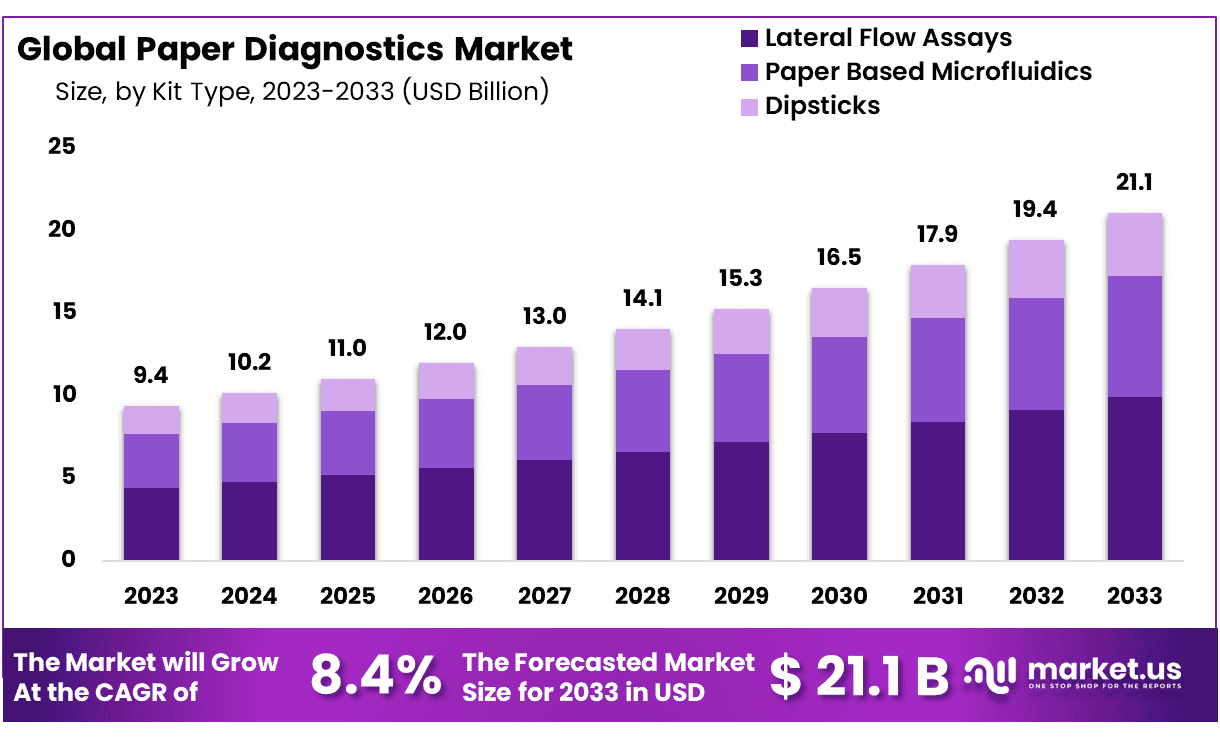
The Global Paper Diagnostics Market size is expected to be worth around USD 21.1 Billion by 2033 from USD 9.4 Billion in 2023, growing at a CAGR of 8.4% during the forecast period from 2024 to 2033.
Paper diagnostics offer a set of cost-effective and user-friendly diagnostic procedures, making them especially valuable in regions where access to more advanced and expensive diagnostic methods may be limited. They serve as a practical alternative in remote areas, where the availability of highly trained medical personnel might be scarce. However, in cases where trained staff is readily available, advanced diagnostic equipment remains a valuable option.
The principle behind paper diagnostics is similar to that of litmus or pH paper. These diagnostics rely on a chemical reaction between the paper’s coating and the substance being tested, resulting in a visible outcome used for diagnosis. In essence, paper diagnostics function much like litmus or pH paper. What sets them apart is their user-friendliness, as they provide clear instructions on how to interpret each visible result. This feature makes paper diagnostics accessible even to individuals with minimal medical expertise, especially for diseases requiring regular self-diagnosis.
Because of the growing demand for affordable healthcare in developing countries, point-of-care diagnostic methods are becoming more popular. Increased adoption of point-of-care pregnancy and diabetes test kits is expected to benefit the market in developed countries. Changing lifestyle habits like smoking and eating unhealthy foods can increase lifestyle-related diseases like diabetes and heart disease.
Key Takeaways
- Market Size: Paper Diagnostics Market size is expected to be worth around USD 21.1 Billion by 2033 from USD 9.4 Billion in 2023.
- Market Growth: The market growing at a CAGR of 8.4% during the forecast period from 2024 to 2033.
- Kite Type Analysis: Lateral Flow Assays emerging as the frontrunner, commanding a substantial 47% market share.
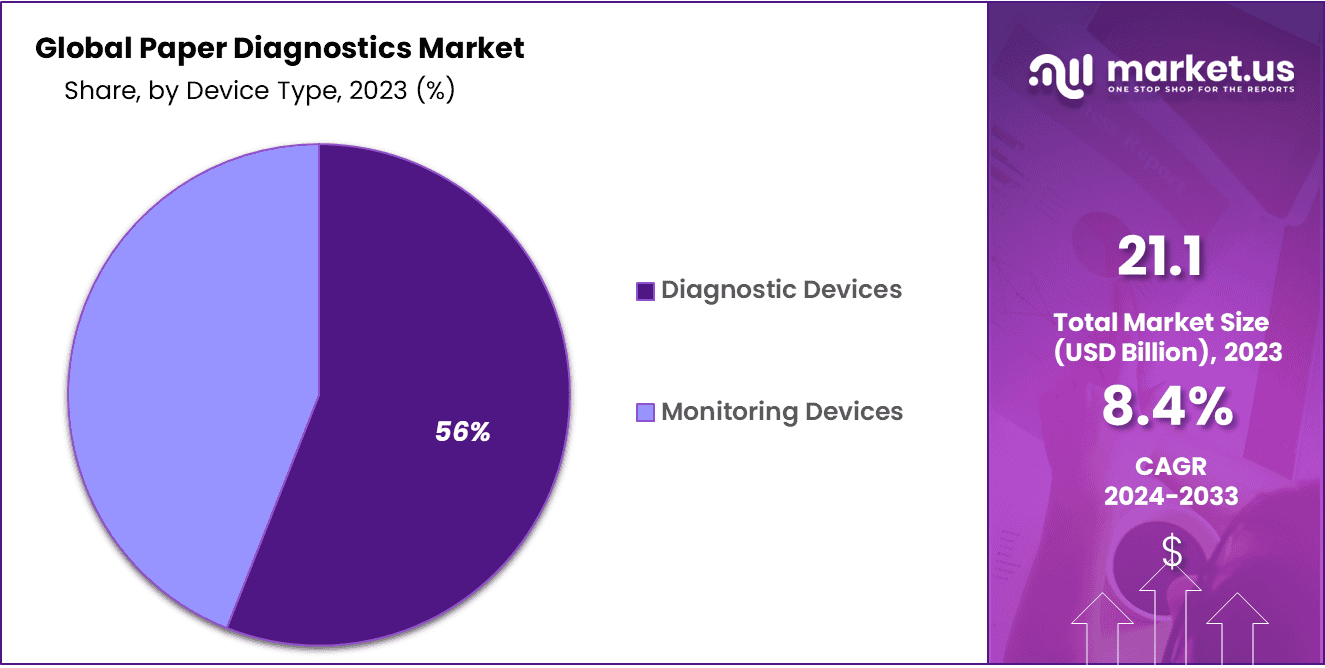
- Device Type Analysis: Diagnostic Devices take center stage, accounting for a substantial 56% of the market share.
- Application Analysis: Clinical diagnostics segment is poised for substantial growth throughout the forecast period.
- End-Use Analysis: Hospitals & Clinics are at the forefront, commanding a substantial 48% market share.
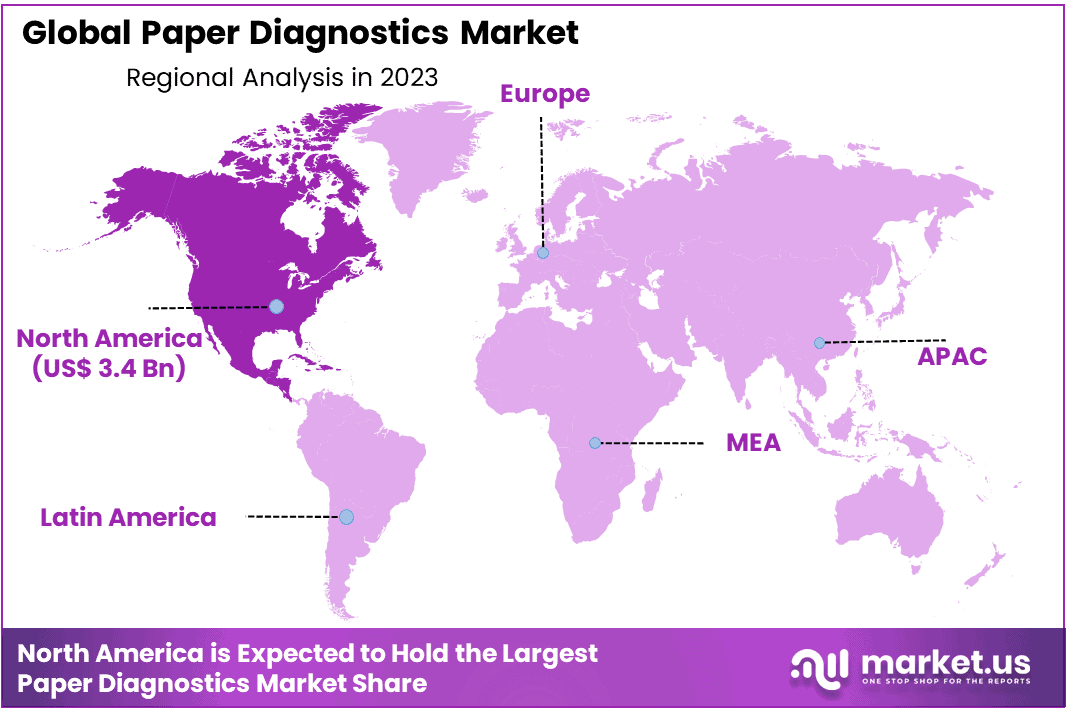
- Regional Analysis: North America dominated the market in 2023, claiming a significant market share of 38% and holding USD 3.4 billion market revenue.
Kite Type Analysis
In the realm of paper diagnostics, the Paper Diagnostics Market is witnessing a significant paradigm shift, with Lateral Flow Assays emerging as the frontrunner, commanding a substantial 47% market share. This dominance is indicative of the growing reliance on these versatile assays, known for their simplicity, rapid results, and cost-effectiveness.
In 2021, the World Health Organization (WHO) reported that tuberculosis (TB) claimed the lives of 1.6 million individuals worldwide, ranking it as the 13th leading cause of death and the second most prominent infectious disease responsible for mortality. Additionally, a staggering 10.6 million people received TB diagnoses during the same year. This data underscores the global impact of TB as a public health concern.
The concerted endeavors of governments and healthcare organizations to raise awareness regarding TB detection and treatment have played a pivotal role in fostering growth within this sector. These concerted efforts are aimed at reducing the burden of TB and improving access to timely diagnosis and effective treatment, ultimately contributing to better health outcomes for affected individuals.
Paper-based microfluidics, particularly in the form of dipsticks, has become a focal point in this transformative landscape. These portable, user-friendly devices are revolutionizing point-of-care testing by harnessing the capillary action within paper to transport fluids, enabling the precise detection of various analytes.
Device Type Analysis
In the dynamic landscape of the Paper Diagnostics Market, Diagnostic Devices take center stage, accounting for a substantial 56% of the market share. This dominance underscores the pivotal role of these devices in revolutionizing healthcare and diagnostics.
Diagnostic Devices in the realm of paper diagnostics encompass a wide array of tools and technologies designed for accurate and rapid disease detection. Leveraging cutting-edge paper-based microfluidics and other innovative techniques, these devices provide healthcare professionals and patients alike with accessible, cost-effective, and reliable diagnostic solutions.
Monitoring Devices also play a crucial role in this evolving market. They offer continuous real-time tracking of various health parameters, enabling proactive healthcare management. These devices are instrumental in remote patient monitoring, chronic disease management, and wellness tracking, catering to the growing demand for personalized healthcare.
The Paper Diagnostics Market’s landscape is marked by its commitment to improving healthcare accessibility, affordability, and effectiveness. As Diagnostic and Monitoring Devices continue to advance, they hold the promise of reshaping the future of healthcare, with a focus on early detection and proactive health management.
Application Analysis
In application analysis, the clinical diagnostics segment is poised for substantial growth throughout the forecast period. This projection is rooted in the escalating utilization of filter paper, microfluidics, and paper-based biosensors for the diagnosis of liver disorders. Furthermore, this segment is expected to maintain its dominant position in the market over the entire forecast horizon, primarily due to the surging incidence of infectious diseases, including but not limited to Ebola, malaria, dengue, tuberculosis, and HIV.
The anticipated growth of the clinical diagnostics segment is further bolstered by significant factors such as new product launches and technological advancements. As a notable example, in November 2022, LumiraDx introduced a rapid microfluidic immunoassay C-Reactive test in India. This innovative product is aimed at curbing antibiotic prescriptions, thereby mitigating the risk of antimicrobial resistance.
Similarly, in April 2017, Alere unveiled “Malaria Ag.P.F,” an exceptionally sensitive malaria Rapid Diagnostic Test (RDT) capable of detecting Plasmodium falciparum antigens, including the histidine-rich protein II antigen, even in cases of very low parasitemia. These developments signify a dynamic and progressive landscape within the clinical diagnostics sector.
End Use Analysis
In the ever-evolving Paper Diagnostics Market, it is evident that Hospitals & Clinics are at the forefront, commanding a substantial 48% market share. This dominance underscores the crucial role these healthcare institutions play in adopting and implementing paper-based diagnostic solutions.
Hospitals and clinics are the primary end-users of paper diagnostics, leveraging these innovative tools for rapid and accurate disease detection, patient monitoring, and point-of-care testing. The reliability, cost-effectiveness, and ease of use offered by paper diagnostics have made them indispensable in the clinical setting, enabling healthcare professionals to make informed decisions swiftly.
While Hospitals & Clinics dominate the market, Homecare Settings are gaining momentum. The convenience and portability of paper-based diagnostic devices make them increasingly suitable for use in home settings. Patients and caregivers can now perform tests and monitor health conditions from the comfort of their homes, reducing the burden on healthcare facilities and enhancing patient autonomy.
Кеу Маrkеt Ѕеgmеntѕ
Kit Type
- Lateral Flow Assays
- Paper Based Microfluidics
- Dipsticks
Device Type
- Diagnostic Devices
- Monitoring Devices
Application
Clinical Diagnostics
- Cancer
- Liver Disorders
- Infectious Diseases
- Others
Environmental Monitoring
Food Quality Testing
End-Use
- Hospital and Clinics
- Homecare Settings
- Other End-Uses
Driver
Growing Prevalence of Infectious Diseases
- The rise in infectious diseases such as HIV, malaria, and COVID-19 has propelled the demand for quick and cost-effective diagnostic solutions.
- Paper diagnostics offer a rapid and affordable means of detecting these diseases, driving market growth.
- In-vitro diagnostics devices and tests have seen a rise in investment by governments. The European Diagnostic Manufacturers Association, which focuses on developing and growing in vitro Diagnostic Industry in Europe each year, invests around EUR 1,000 million in R&D in vitro diagnostics.
Cost-Effectiveness and Accessibility
- Paper-based diagnostic tests are less expensive to produce and distribute compared to traditional lab tests, making them accessible to a wider population.
- This affordability factor has a significant impact on market expansion.
Trend
Advancements in Sensing Technologies
- Innovations in sensing technologies, such as microfluidics and nanoparticles, are enhancing the sensitivity and accuracy of paper-based diagnostic tests.
- These advancements contribute to the market’s continuous growth.
Point-of-Care Testing (POCT) Adoption
- The shift towards decentralized healthcare and the need for on-the-spot diagnosis has led to the increasing adoption of Point-of-Care Testing.
- Paper diagnostics align perfectly with this trend, providing immediate results.
Restraint
Limited Diagnostic Capabilities
- Paper diagnostics may have limitations in terms of the range of diseases they can detect and the complexity of diagnostic procedures.
- Some complex medical conditions may require more sophisticated lab-based tests, posing a constraint on market expansion.
Quality Control Challenges
- Ensuring the consistency and reliability of paper-based tests across different batches can be challenging.
- Maintaining strict quality control standards is imperative to overcome this restraint.
Opportunity
Emerging Markets Growth
- Emerging economies with large populations are witnessing increased adoption of paper diagnostics due to their cost-effectiveness and ease of use.
- Expanding into these markets presents a significant growth opportunity.
Customized Test Kits
- Tailoring paper-based diagnostic kits for specific diseases or regions with unique healthcare needs can open up niche markets.
- Customization offers a chance to cater to specialized demands.
Regional Analysis
By geographical distribution, North America dominated the market in 2023, claiming a significant market share of 38% and holding USD 3.4 billion market revenue. This region is expected to maintain a consistent trend throughout the forecast period. The growth in North America can be attributed to several factors, including the presence of key market players, academic and research institutes, and a rising incidence of infectious diseases.
Notably, engineers at MIT have developed an affordable and user-friendly paper-based diagnostic test, enhancing diagnosis rates and enabling early-stage treatment. Likewise, a biochemist from the University of Washington has created a handheld plastic device.
also, the Asia Pacific market is poised to exhibit the most rapid Compound Annual Growth Rate (CAGR) at 7.8% over the forecast period. This surge can be attributed to increased investments in the healthcare sector, government initiatives, advancements in education, and heightened awareness of health-related matters.
Within the Asia Pacific region, the Indian market is anticipated to experience the highest CAGR during the forecast period. This growth is underpinned by a surging demand for cost-effective diagnostic tools tailored to rural populations and the acceleration of product development by local companies.
Key Regions and Countries
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Russia
- Spain
- Rest of Europe
- APAC
- China
- Japan
- South Korea
- India
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- MEA
- GCC
- South Africa
- Israel
- Rest of MEA
Key Players Analysis
ARKRAY Inc., Acon Laboratories, Inc., Bio-Rad Laboratories, and Siemens Healthcare GmbH are key players. These players have adopted high R&D investments to create new product portfolios. This is one of their key strategies to gain shares. In FY 2017, Abbott spent USD 2,240 million on research and development activities. The company also has HIV Combo Fingersticks, SD BIOLINE HAT 2.0, and Malaria Ag The company offers a range of test products to the market.
In 2018, ARKRAY, Inc. also completed the expansion of and transfer to its Pinghu plant in China. This was done to increase its manufacturing capabilities for diabetes testing instruments as well as in vitro diagnostic reagents. The company plans to expand its China presence through R&D unit expansion.
Other emerging players, including Abcam Plc, Chembio Diagnostic Systems, Inc., and Creative Diagnostics, continue to focus on product development and expand their presence in developing countries to seize untapped opportunities.Маrkеt Kеу Рlауеrѕ
- Arkray, Inc.
- Acon Laboratories, Inc.
- Bio-Rad Laboratories, Inc.
- Alere Inc.
- Gvs S.P.A.
- Diagnostics For All, Inc.
- Siemens Healthcare Gmbh
- Navigene
- Kenosha Tapes
- Micro Essential Laboratory Inc.
- Ffei Life Science (Biognostix)
- Other Key Players
Recent Developments
- Arkray, Inc.(March 2024): Arkray, Inc. launched the GlucoCard Shine, an advanced paper-based glucose monitoring system. The device features enhanced accuracy and user-friendly design, aimed at improving diabetes management through easy and accessible testing solutions.
- Acon Laboratories, Inc.(April 2024): Acon Laboratories, Inc. acquired Biomed Innovations, a leader in paper-based diagnostic technologies. This acquisition is set to bolster Acon’s portfolio in rapid diagnostic testing, expanding their market reach and innovative capabilities in paper diagnostics.
- Bio-Rad Laboratories, Inc.(January 2024): Bio-Rad Laboratories, Inc. introduced the PaperDx Pro, a cutting-edge paper diagnostics platform for infectious diseases. This product leverages advanced biomarker detection techniques, providing quick and reliable results, and is designed to enhance point-of-care diagnostics.
- Diagnostics For All, Inc.(June 2024): Diagnostics For All, Inc. acquired NanoTest Systems, a company specializing in nanotechnology-enhanced paper diagnostics. This acquisition is expected to drive advancements in ultra-sensitive diagnostic tools, expanding the company’s innovative product line.
- Siemens Healthcare Gmbh (March 2024): Siemens Healthcare Gmbh unveiled the Siemens PaperScan, a highly sensitive diagnostic device for early disease detection. This product employs advanced paper microfluidics, offering a non-invasive, cost-effective solution for rapid diagnostics.
- Navigene (April 2024): Navigene merged with BioScreen Technologies to combine their expertise in genetic screening and paper diagnostics. This strategic merger aims to develop comprehensive, paper-based genetic testing solutions, enhancing the accuracy and speed of genetic diagnostics.
Report Scope
Report Features Description Market Value (2023) USD 9.4 Billion Forecast Revenue (2033) USD 21.1 Billion CAGR (2024-2033) 8.4% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Kit Type-(Lateral Flow Assays, Paper Based Microfluidics, Dipsticks); By Device Type-(Diagnostic Devices, Monitoring Devices); By Application-(Clinical Diagnostics(Cancer, Liver Disorders, Infectious Diseases, Others) Environmental Monitoring, Food Quality Testing); By End-Use-(Hospital and Clinics, Homecare Settings, Other End-Uses) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Arkray, Inc., Acon Laboratories, Inc., Bio-Rad Laboratories, Inc., Alere Inc., Gvs S.P.A., Diagnostics For All, Inc., Siemens Healthcare Gmbh, Navigene, Kenosha Tapes, Micro Essential Laboratory Inc., Ffei Life Science (Biognostix), Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What are paper diagnostics?Paper diagnostics refer to a set of diagnostic procedures conducted using cost-effective and user-friendly paper-based instruments. These tests are often used in regions where advanced diagnostic technologies may not be readily available.
How big is the Paper Diagnostics Market?The global Paper Diagnostics Market size was estimated at USD 9.4 Billion in 2023 and is expected to reach USD 21.1 Billion in 2033.
What is the Paper Diagnostics Market growth?The global Paper Diagnostics Market is expected to grow at a compound annual growth rate of 8.4%. From 2024 To 2033
Who are the key companies/players in the Paper Diagnostics Market?Some of the key players in the Paper Diagnostics Markets are Arkray, Inc., Acon Laboratories, Inc., Bio-Rad Laboratories, Inc., Alere Inc., Gvs S.P.A., Diagnostics For All, Inc., Siemens Healthcare Gmbh, Navigene, Kenosha Tapes, Micro Essential Laboratory Inc., Ffei Life Science (Biognostix), Other Key Players.
How do paper diagnostics work?Paper diagnostics operate on the principle of chemical reactions between the paper's coating and the substance being tested. This reaction produces visible results that can be used for diagnosis, similar to litmus or pH paper.
Where are paper diagnostics commonly used?Paper diagnostics are frequently employed in remote or resource-constrained areas where access to advanced diagnostic equipment and trained staff is limited.
What advantages do paper diagnostics offer?Paper diagnostics offer cost-effective and accessible diagnostic solutions, allowing even inexperienced individuals to interpret results due to clear instructions provided.
Are paper diagnostics suitable for self-diagnosis?Yes, paper diagnostics are suitable for self-diagnosis, making them valuable for individuals who need to regularly monitor their health conditions.

-
-
- Arkray, Inc.
- Acon Laboratories, Inc.
- Bio-Rad Laboratories, Inc.
- Alere Inc.
- Gvs S.P.A.
- Diagnostics For All, Inc.
- Siemens Healthcare Gmbh
- Navigene
- Kenosha Tapes
- Micro Essential Laboratory Inc.
- Ffei Life Science (Biognostix)
- Other Key Players










