Global Immune Thrombocytopenia Market by Type (Acute Immune Thrombocytopenia, and Chronic Immune Thrombocytopenia), By Treatment (Intravenous Immunoglobulins, Corticosteroids, and Thrombopoietin Receptor Agonists), By End-User (Hospitals and Clinics, Specialty Centers, and Research and Academic Institutes), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 102400
- Number of Pages: 322
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
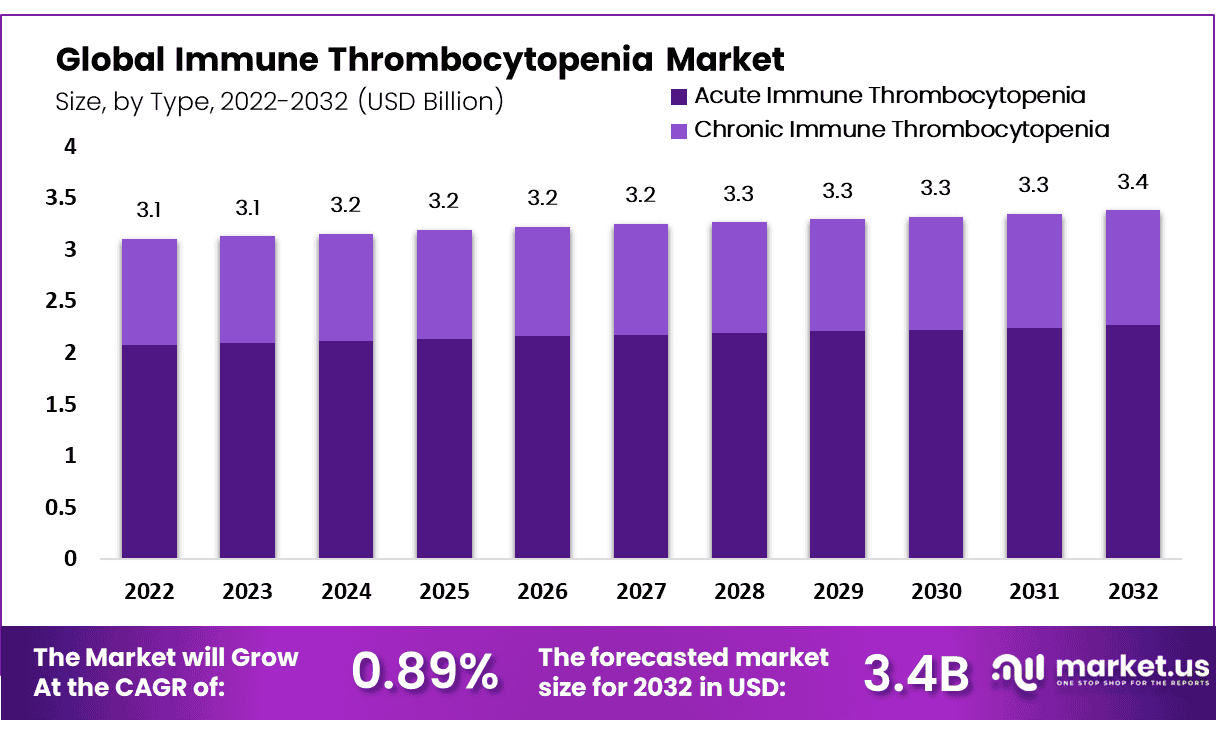
Immune Thrombocytopenia Market size is expected to be worth around USD 3.4 Billion by 2032 from USD 3.1 Billion in 2022, growing at a CAGR of 0.89% during the forecast period from 2023 to 2032.
Low platelets count in immune thrombocytopenia is an autoimmune condition that can cause bleeding and brushing. In coming years, the market for immune thrombocytopenia is anticipated to grow significantly owing to the several numbers factor such as rising IIP prevalence, growing public awareness of the condition as well as incising efforts in research and development to build new treatments for this type of disease. Immune Thrombocytopenia also known as idiopathic thrombocytopenic purpura is a rare blood condition that is characterized by an exceptionally low platelet count and results in excessive bleeding and wound.
Due to greater awareness also regular healthcare check-ups in both developed and developing countries, ITP is being diagnosed more frequently. As this ITP can only be identified through a normal blood examination, there is an urgent need to increase awareness of it. The current state of the market offers favorable conditions for using immune thrombocytopenia as a treatment. As a second line of treatment for the illness, TPO-RA is widely regarded. A rise in immunological thrombocytopenia and the launch of new products are some of the variables that are expected to contribute to the expansion of idiopathic thrombocytopenia.
Key Takeaways
- Market Growth: Projected to reach USD 3.4 billion by 2032, with a CAGR of 0.89% from 2023 to 2032.
- Prevalence: Rising ITP cases globally, impacting all age groups, driving demand for effective treatments.
- Diagnostic Advancements: Improved diagnostic tools enhance accuracy, prompting early detection and management, boosting market growth.
- Awareness Impact: Increased awareness among patients and healthcare professionals leading to higher diagnosis rates.
- Innovative Therapies: Development of novel ITP treatments fuels market expansion and enhances patient outcomes.
- Restraints: Underdiagnosis persists due to lack of awareness; treatment costs and side effects limit accessibility.
- Targeted Therapies Opportunity: Potential for more precise treatments addressing ITP’s immune dysregulation, driving innovation.
- Digital Health Integration: Telemedicine and digital tools enhance patient engagement, offering efficient and remote ITP management.
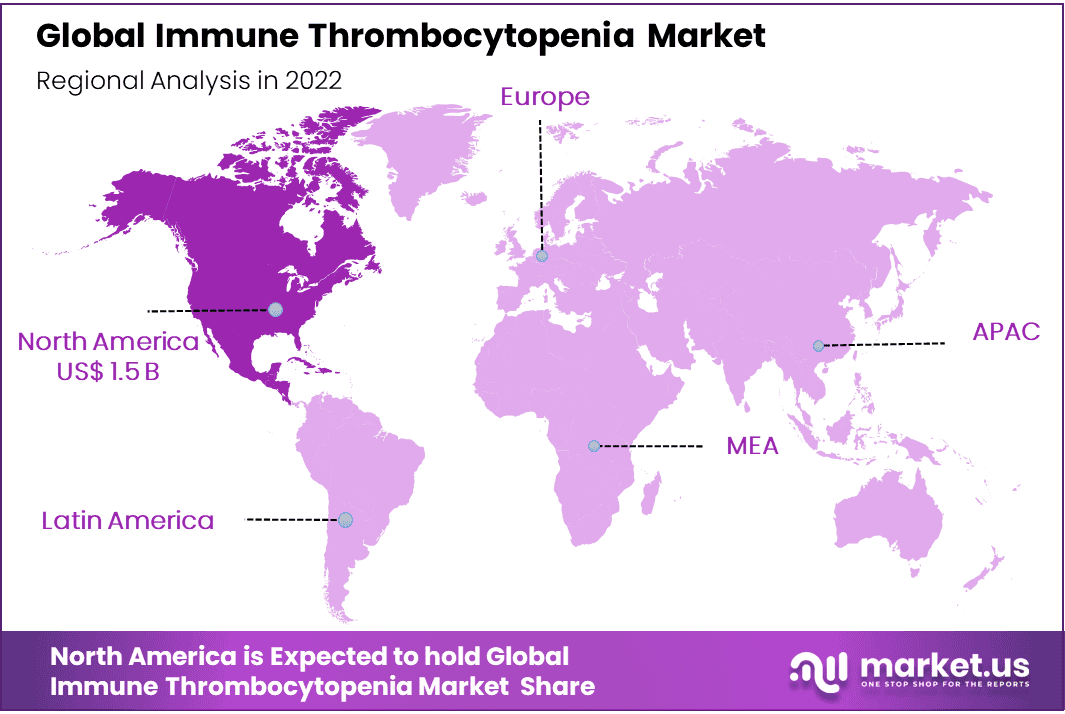
- Regional Dominance: North America leads with 48% market share, driven by advanced healthcare infrastructure and research.
- Fastest-Growing Region: Asia-Pacific, with large patient populations, experiences rapid growth, fueled by healthcare investments.
Driving Factors
The rising prevalence of ITP worldwide is a significant driver of the market. ITP affects individuals of all ages and genders, and the increasing incidence of autoimmune disorders contributes to the growing patient population with ITP, thus driving the demand for effective treatments. Technological advancements in diagnostic tools and techniques have improved the accuracy and efficiency of ITP diagnosis. This enables early detection and appropriate management, leading to increased demand for diagnostic tests and supporting the growth of the market.
Increased awareness of ITP among patients, healthcare professionals, and the general population has led to improved diagnosis rates. Greater recognition and understanding of the condition prompt individuals to seek medical help, contributing to the market’s growth. The development of novel treatment options and therapies for ITP is a significant driver. Thrombopoietin receptor agonists immunosuppressive drugs, and newer therapeutic modalities offer improved outcomes and better management of ITP, increasing treatment options and driving market growth.
Ongoing research and development efforts focused on ITP contribute to the expansion of treatment options and the discovery of novel therapies. Investments in clinical trials, drug development, and collaborations between academic institutions, pharmaceutical companies, and regulatory bodies drive the market by fostering innovation.
Restraining Factors
Despite growing awareness, ITP remains underdiagnosed and underreported in many regions. Lack of awareness among patients and healthcare professionals about the condition and its symptoms can lead to delayed diagnosis and hinder market growth. The cost of ITP treatment, including medications, diagnostic tests, and supportive care, can be a significant restraint for patients and healthcare systems. Expensive therapies and the need for long-term management can limit access to optimal treatment, especially in regions with limited healthcare resources. Immunosuppressive medications and some corticosteroid therapies for ITP may have serious side effects. Patient acceptability and treatment compliance may be affected by worries about side effects and possible risks associated with prolonged use which may constrain the market.
Despite advancements in treatment options, there is still a need for more targeted therapies that specifically address the underlying immune dysregulation in ITP. The absence of highly selective treatments limits the ability to achieve complete and sustained responses in all patients, impacting the market’s growth potential. ITP is a heterogeneous disorder with variations in disease presentation, response to treatment, and disease course among individuals. This heterogeneity poses challenges in developing standardized treatment approaches and personalized management strategies, which can be a restraint for the market.
Growth Opportunities
There is an opportunity for the development of more targeted therapies that address the underlying immune dysregulation in ITP. Novel treatment options focusing on specific immune targets and pathways have the potential to improve treatment outcomes and offer personalized approaches to managing the condition. The ITP market can benefit from the expansion of healthcare infrastructure and increased access to medical facilities in emerging markets. These regions, with growing populations and improving healthcare systems, present opportunities for market expansion and the introduction of new treatment options.
Partnerships between pharmaceutical companies, academic institutions, and research organizations can drive innovation in ITP treatment. Collaborative efforts such as clinical trials, sharing knowledge among colleagues, and exploring novel therapies may result in more effective and safe treatments being developed. Early identification and diagnosis of ITP may benefit from enhanced patient education and awareness programs, which increase the probability of correct diagnosis, ensure prompt treatment and improve outcomes by increasing awareness among both healthcare providers and their patients of its symptoms.
Telemedicine and digital health tools used in ITP management can increase patient participation, facilitate remote monitoring, and expand access to healthcare services. Telehealth platforms and digital tools make this possible while helping to enhance care delivery without geographical limitations being an obstacle.
Trending Factors
There is an increasing emphasis on personalized medicine approaches in ITP treatment. Advancements in molecular profiling, biomarker research, and genetic testing are facilitating the identification of patient subgroups and enabling tailored treatment strategies. This trend aims to optimize treatment outcomes and enhance patient care by customizing therapies based on individual patient characteristics. Thrombopoietin Receptor Agonists (TPO-RAs) are gaining significant traction in the ITP market. These agents stimulate platelet production and have demonstrated efficacy in increasing platelet counts and reducing bleeding symptoms. The growing adoption of TPO-RAs as second-line or subsequent-line therapies reflects their positive impact on disease management and their potential to improve patient outcomes.
Biologics and novel therapies have become a trend in ITP markets, as researchers and pharmaceutical companies research new treatment modalities such as monoclonal antibodies, immune modulators, targeted therapies, or immunotherapies to offer patients who refractory to traditional treatments or experience relapses an alternative option for care.
Digital health solutions are being increasingly integrated into ITP management. Mobile applications, wearable devices, and telemedicine platforms are facilitating remote monitoring, patient engagement, and adherence to treatment plans. These technologies enhance convenience, improve patient-provider communication, and enable real-time data tracking, leading to more efficient and patient-centric care. Healthcare providers and pharmaceutical companies have increasingly acknowledged the significance of patient-centric care for ITP sufferers and its effect on the quality of life. Healthcare providers and pharmaceutical companies are placing increased emphasis on treating not only physical symptoms associated with living with ITP but also its psychosocial aspects. Efforts to improve patient education, support groups, and access to comprehensive care are trends that prioritize the holistic well-being of individuals with ITP.
Type Analysis
The Acute Immune Thrombocytopenia Segment Accounted for the Largest Revenue Share in the Global Immune Thrombocytopenia Market in 2022.
Based on type, the market for immune thrombocytopenia is segmented into acute immune thrombocytopenia and chronic immune thrombocytopenia. Acute immune thrombocytopenia dominates the market with 67% of the market share. Acute immune thrombocytopenia known as acute idiopathic thrombocytopenia purpura is a sudden onset autoimmune disorder characterized by low platelet count. The exact cause is unclear but it often follows a viral infection or can be triggered by medicines. Acute ITP presents with symptoms like petechiae, easy bruising, and bleeding, potentially leading to severe complications.
Diagnosis involves a medical history, physical examination, and blood tests showing thrombocytopenia without other underlying causes. While children tend to experience favorable outcomes with spontaneous remission within six months, adults may have a more variable course. The management of acute ITP depends on the severity of symptoms and platelet count. In mild cases, close observation without specific treatment may be sufficient. Treatment options may include corticosteroids to suppress the immune response, intravenous immunoglobulin (IVIG) to temporarily increase platelet counts, and, in rare cases, platelet transfusions or surgical removal of the spleen (splenectomy).
Treatment Analysis
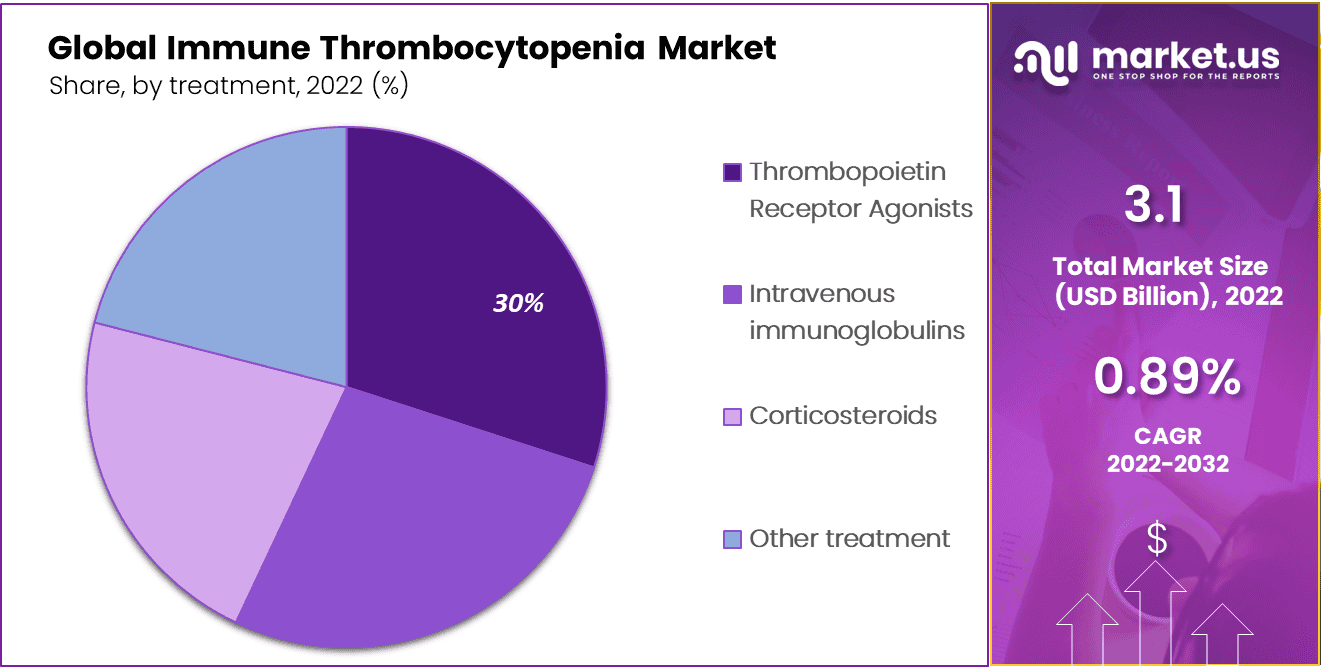
The Thrombopoietin Receptor Agonists (TPO-RAs) Segment Accounted for the Largest Revenue Share in the Global Immune Thrombocytopenia Market in 2022.
By treatment, the market is further divided into thrombopoietin receptor agonists, intravenous immunoglobulins, corticosteroids, and other treatments. The thrombopoietin receptor agonists (TPO-RAS) dominated the market with 30% of the market share. The treatment of immune thrombocytopenia (ITP) involves evaluating various factors. Firstly, it entails assessing different types of TPO-RAs available in the market, such as romiplostim and eltrombopag, to understand their specific characteristics, efficacy, and safety profiles.
Secondly, the mode of administration is considered, as TPO-RAs can be administered orally or through subcutaneous injections, which impacts patient preferences and convenience. Additionally, the treatment line analysis determines the position of TPO-RAs in the ITP treatment algorithm, specifically as a second-line or subsequent-line therapy after initial treatments. Segmenting the pediatric and adult populations helps to address age-specific considerations, dosing, safety, and efficacy data.
End-User Analysis
The Hospitals and Clinics Segment Accounted for the Largest Revenue Share in the Global Immune Thrombocytopenia Market in 2022.
Based on end-user, the market is segmented into hospitals and clinics, specialty centers, research, and academic institutes. Among these end-users, the hospitals and clinics segment dominated the market with 54% of the market share. Due to the increased use of intravenous immunoglobulin and TPO-RA treatments for ITP. In addition, a rise in the use of rescue therapy in hospitals is caused by a higher frequency of bleeding disorders in people with immune thrombocytopenia.
Hospital pharmacies are able to dominate the ITP market due to this feature as well. Due to the growing tendency towards oral therapeutic treatments and the purchase of the same through retail channels, retail pharmacy is anticipated to see a sizable CAGR in the worldwide ITP market. However, because of their relatively lower adoption in the Middle East, Africa, and South America, the other (online pharmacies and mail-order pharmacies) sector is predicted to hold a smaller part of the market.
Key Market Segments
Based on Type
- Acute Immune Thrombocytopenia
- Chronic Immune Thrombocytopenia
Based on Treatment
- Intravenous Immunoglobulins
- Corticosteroids
- Thrombopoietin Receptor Agonists
- Other Treatment
Based on end users
- Hospitals and Clinics
- Specialty Centers
- Research and Academic Institutes
Regional Analysis
North America Accounted for the Largest Revenue Share in the Global Immune Thrombocytopenia Market in 2022.
North America dominates the global market with 48% of the market share. North America is a significant market for ITP, driven by well-established healthcare infrastructure, increased awareness, and advanced diagnostic and treatment options. The region has a strong presence of key pharmaceutical companies and research institutions actively engaged in ITP research and development. Additionally, favorable reimbursement policies and robust regulatory frameworks contribute to market growth.
Asia-Pacific is Expected as Fastest Growing Region in Projected Period in Global Immune Thrombocytopenia Market.
The Asia Pacific region is witnessing significant growth in the ITP market due to factors such as a large patient population, improved healthcare infrastructure, and rising awareness. Developing countries like China and India are key contributors to the market expansion. The presence of a large number of pharmaceutical manufacturers and increasing investments in healthcare further drive market growth in this region.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Market shares of specific companies can vary over time and may depend on factors such as the availability and adoption of their respective therapies, regional market dynamics, and competitive landscape. Market share analysis is often conducted through comprehensive market research and may require access to specific industry reports or databases for the most up-to-date information on market share distribution among key players in the Global ITP market.
Market Key Players
Listed below are some of the most prominent bio-based surface disinfectant industry players.
- Pfizer Inc.
- Hoffmann-La Roche Ltd
- Mylan N.V.
- Fresenius Kabi AG
- Hikma Pharmaceuticals PLC
- Hepalink Group.
- Teva Pharmaceutical Industries Ltd.
- Amarillo Biosciences Inc
- Bolder Bio Technology Inc.
- GENOSCO Inc.
- Hansa Biopharma
- Janssen Pharmaceuticals Inc.
- Eisai Co Ltd
- CSL Limited
- Amgen Inc.
- Kyowa Hakko Kirin Co. Ltd.
- Rigel Pharmaceuticals Inc.
- Shionogi Inc.
- Dova Pharmaceuticals
- Novartis AG
- Jiangsu Hengrui Pharmaceutical Co. Ltd.
- Shire and Ligand Pharmaceuticals Inc.
- Other Key Players
Recent Developments
- In July 2019, Intas Pharmaceuticals made a ground-breaking move to increase access to treatment for persistent immune thrombocytopenia. Under the brand name Romy, the business introduced Romiplostim in India.
- In December 2018, FDA approved Amgen’s Nplate (Romiplostim) medication. It is meant to treat young patients with immune thrombocytopenia who are at least one year old.
Report Scope
Report Features Description Market Value (2022) USD 3.1 Bn Forecast Revenue (2032) USD 3.4 Bn CAGR (2023-2032) 0.89% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered Based on Type (Acute Immune Thrombocytopenia, Chronic Immune Thrombocytopenia); Based on Treatment (Intravenous Immunoglobulins, Corticosteroids, Thrombopoietin Receptor Agonists, Other Treatment); Based on end users (Hospitals and Clinics, Specialty Centers, Research and Academic Institutes) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; the Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Pfizer Inc., Hoffmann-La Roche Ltd, Mylan N.V., Fresenius Kabi AG, Hikma Pharmaceuticals PLC, Hepalink Group., Teva Pharmaceutical Industries Ltd., Amarillo Biosciences Inc, Bolder Bio Technology Inc., GENOSCO Inc., Hansa Biopharma, Janssen Pharmaceuticals Inc., Eisai Co Ltd, CSL Limited, Amgen Inc., Kyowa Hakko Kirin Co. Ltd., Rigel Pharmaceuticals Inc., Shionogi Inc., Dova Pharmaceuticals, Novartis AG, Jiangsu Hengrui Pharmaceutical Co. Ltd., Shire and Ligand Pharmaceuticals Inc., Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the Immune Thrombocytopenia Market Size During the Forecast Year 2032?The Global Immune Thrombocytopenia Market size is expected to be worth around USD 3.4 Billion by 2032 growing at a CAGR of 0.98%.
What is the Immune Thrombocytopenia Market CAGR During the Forecast Period?The Global Immune Thrombocytopenia Market size is growing at a CAGR of 0.98% during the forecast period from 2023 to 2032.
What was the Immune Thrombocytopenia Market Size in the Year 2022?The Global Immune Thrombocytopenia Market size was USD 3.1 Billion in 2022, growing at a CAGR of 0.98% during the forecast period from 2023 to 2032.
 Immune Thrombocytopenia MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Immune Thrombocytopenia MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Pfizer Inc.
- Hoffmann-La Roche Ltd
- Mylan N.V.
- Fresenius Kabi AG
- Hikma Pharmaceuticals PLC
- Hepalink Group.
- Teva Pharmaceutical Industries Ltd.
- Amarillo Biosciences Inc
- Bolder Bio Technology Inc.
- GENOSCO Inc.
- Hansa Biopharma
- Janssen Pharmaceuticals Inc.
- Eisai Co Ltd
- CSL Limited
- Amgen Inc.
- Kyowa Hakko Kirin Co. Ltd.
- Rigel Pharmaceuticals Inc.
- Shionogi Inc.
- Dova Pharmaceuticals
- Novartis AG
- Jiangsu Hengrui Pharmaceutical Co. Ltd.
- Shire and Ligand Pharmaceuticals Inc.
- Other Key Players









