Global HERG Screening Market by Type (Gene KCNH2, Mutant KCNH2), By ION Channel (Voltage-Gated Ion Channel, Ligand-Gated Ion Channel, mechanosensitive, Transient receptor potential), By Application (Antiarrhythmic, Antipsychotic, Antibiotic, Antimalarial, Antihistamine) By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2024-2033
- Published date: March 2024
- Report ID: 83472
- Number of Pages: 320
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
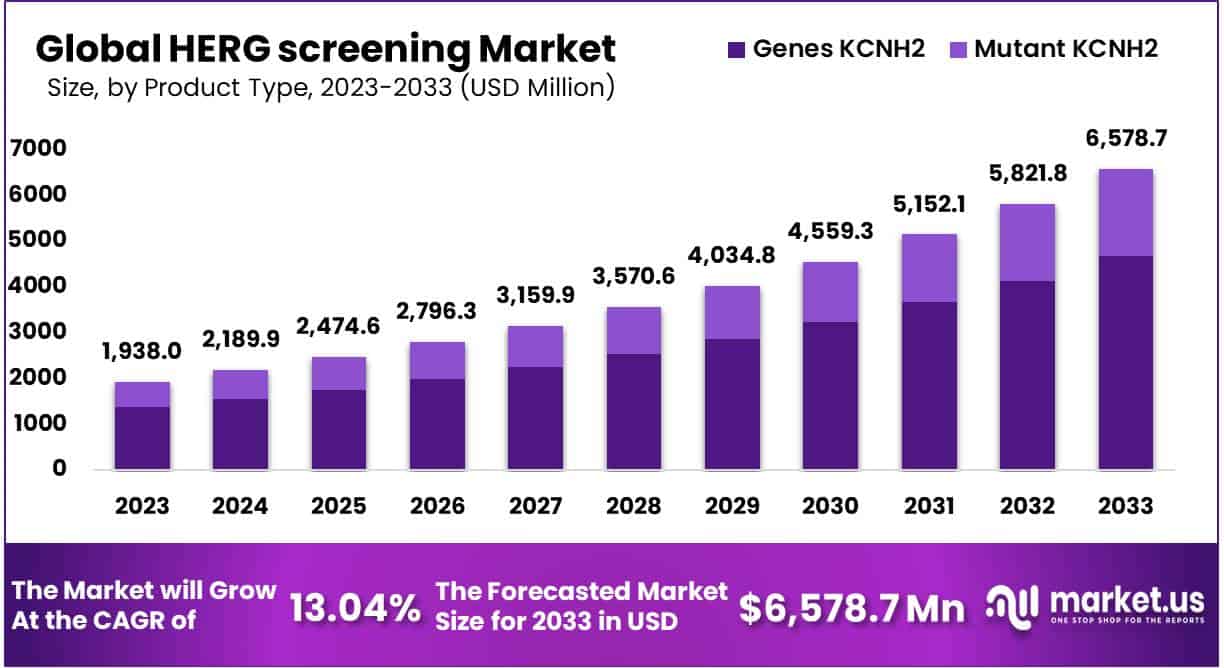
The Global HERG Screening Market size is expected to be worth around USD 6578.7 Million by 2033 from USD 1,938.0 Million in 2023, growing at a CAGR of 13.04% during the forecast period from 2024 to 2033.
The protein Kv11.1, alpha subunit of potassium ion channel is coded by a gene known as Ether a-go-go Related Gene or hERG. The repolarizing current in the cardiac action potential is moderated by potassium ion channel, helping in cardiac pulsation. hERG screening is primarily used in drug discovery process. Before the submission of investigational new drug, hERG assessment is highly prioritized by the regulatory agencies. Microelectrode array and Fluorescence polarization assay are the different assays of hERG screening, making it very accurate and quick.
As a component of cardiac safety and pharmacology, hERG is playing a crucial role for past many years. The elevated prices of products used in HERG screening is expected to hampers the market growth during the forecast period. However, the rise in biotechnology and pharmaceutical companies, rise in prevalence of heart illnesses and untapped market are likely to set lucrative opportunities for its market expansion.
Key Takeaways
- According to product type analysis, HERG screening market is segmented into gene KCNH2 and mutant KCNH2, where genes KCNH2 dominated the product type segment.
- Voltage gated, ligand gated, mechanosensitive and transient receptor potential are various ion channels under ION channel analysis.
- Antiarrhythmic segment is the major contributor to the market growth of hERG screening, as various diseases are treated with antiarrhythmic drugs.
- North America is the largest market share holder of hERG screening, dominating Europe and APAC regions.
- The major factor driving market growth of hERG screening is rising number heart illnesses.
- High cost of the products used in screening is expected to impede the market growth in the subsequent period.
Product Type Analysis
Genes KCNH2 dominates the mutant KCNH2
Based on type analysis, HERG screening market is divided into two segments: Genes KCNH2 and Mutant KCNH2. KCNH2 genes dominate the product type segment as it is accounted to hold the largest market share of 71.2% in 2023.These channels help in transporting positively charged atoms of potassium out of the cells, making the cells capable of generating and transmitting electric signals. Channels consisting KCNH2 genes are active in heart muscles, involved in recharging the heart muscles after each heartbeat to maintain a regular rhythm.
The other group of genes are the mutant KCNH2 genes that cause a heart condition called short QT Syndrome, responsible for the cardiac muscle to take less time than usual to recharge between beats. This causes abnormal heart rhythm leading to sudden death.
ION Channel Analysis
Voltage gated ion channel to exhibit major contribution to the market
Based on ION channel Analysis, the hERG Screening market is bifurcated into voltage gated ion channels, ligand gated ion channels, Mechanosensitive ion channels and Transient receptor potential channels. Amongst all these segments, voltage gated ion channels makes large contribution of 68.03%, dominating the market of HERG screening in 2023, with a CAGR of 14.9% during the forecast period. Voltage gated ion channel family members such as Na+, Ca+ and K+ mediate the opening and closing of cell membrane so that transit of ion across cell membrane takes place. Muscle skeletal cells, neurons, heart cells depend on these ion channels to maintain the ionic gradient necessary for cellular functions.
Also, voltage gated ion channel are extensively used to treat disorders like epilepsy and neuropathic pain. All these factors contribute to the growth of voltage gated ion channel market size. On the other hand, ligand gated ion channel contributes to conformational alterations that open channel gates and let ion inflow across the plasma membrane.
Rising number of pharmaceutical and biotechnology companies outsourcing ion channel services and the demand for drug screening for HERG are the key factors contributing to the development of mechanosensitive ion channel and transient potential receptor channels segments. Prevention of tissues functioning abnormally or slowing down the transmission of impulses in tissues that conduct too quickly are done by these medications.
Application Analysis
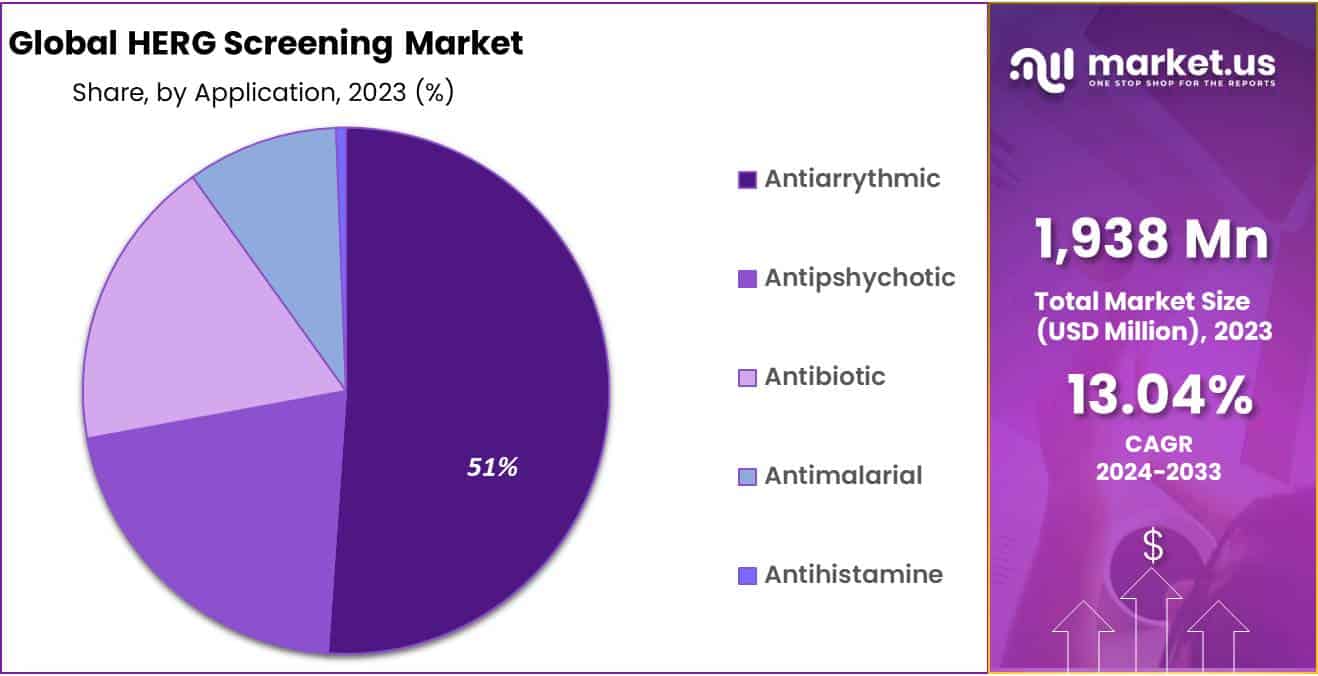
Antiarrythmic segment dominates the HERG screening market
Based on application, hERG screening market is divided into antiarrhythmic, antipsychotic, antibiotics, antimalarial and antihistamine segment. Antiarrhythmic segment is accounted to hold the largest market share, dominating rest of the segments by 51.1% in 2023. There are various medical conditions treated with antiarrhythmic drugs such as premature beats and symptomatic tachycardia. Increase in cardiovascular illnesses, usage of assays for treatment of arrhythmia and safety and efficacy of HERG screening leads to the market expansion.
Another factor expanding the hERG screening market is the rise in number of patients with anxiety, bipolar disorders, and case studies conducted by researchers on antipsychotic drugs using hERG channels. For treating specific mental health issues including psychotic experiences, antipsychotic drugs are approved.
The growing usage of sparfloxacin in drug discovery process, also employed for hERG screening drives the market growth. These antibiotics are also utilized as a positive control in testing the ability of new medications to lengthen the QT interval. The drawback lies where these antibiotics blocks the HERG potassium K+ channel, hence restricting the market growth. Further, antimalarial and antihistamine segment is giving a hand to boost the market growth of HERG screening.
Key Market Segments
By Type
- Gene KCNH2
- Mutant KCNH2
By ION channel
- Voltage gated ion channel
- Ligand gated ion channel
- Mechanosensitive ion channel
- Transient receptor potential channel
By Application
- Antiarrhythmic
- Antipsychotic
- Antibiotic
- Antimalarial
- Antihistamine
Drivers
Increase in number of Cardiovascular diseases drive the HERG screening market
The rising incidences of cardiovascular diseases drive the market of HERG screening in 2023. Dormant lifestyles, hypertension, diabetes, hypercholesterolemia are the main reasons for cardiovascular illnesses. Every 40 seconds, an American suffers from myocardial infarction, according to American College of Cardiology. In 2017, 3,56,461 Americans experienced cardiac arrest outside of a hospital, according to emergency medical services. Amongst them only 8.2% of people had proper functional status. All these factors leads to the development of drug discovery, hence will assist in fostering the market of HERG screening.
Other driving factors that leads to market expansion are increase in R&D for new product launches, increase in approvals for new drug entities, growing demand for drug formulations and development of drugs to treat diseases. Also, ongoing expansion of pharmaceutical companies leads to more usage of HERG screening during drug development, as it is a critical step to ensure patient safety.
Restraints
High cost of products used in screening
The cardiac safety evaluation is one of the crucial step performed during nonclinical drug development. This safety evaluation is done through HERG screening which displays better results, as cardiac activity is one of the vital function of the body. But the cost of products used in screening are high due to usage of modern technology. For Instance, the Eurofins DiscoverX Corporation sells precision recombinant HERG potassium ion channel membrane preparation for the USD 991 per vial. Also, due to negative market growth, the price of vial used in HERG screening is high. Therefore, these factors are anticipated to limit the market extension.
In 2005, International Conference on Harmonization of technical requirements for registration of pharmaceuticals for Human use (ICH) S7B guidance lays out a nonclinical testing strategy to access cardiac risks with two components. The first is an in vitro assay of current through HERG (also known as KCNH2) potassium channel and second is an in vivo assay in an animal model. Typically, a HERG functional current assay costs about the US$ 20,000 and an in vivo assay in dogs can cost US$ 1,00,000. Hence, limiting the market expansion.
Lack of qualified professionals
Cardiotoxicity screening operations require skilled professionals, as this operation is a very critical step to know cardiotoxicity in patients. To increase efficacy of cardiotoxicity screening these professionals are in high demand. But lamentably there is a lack of qualified and trained professionals, restraining the market growth of hERG screening.
Opportunities
Medication Disclosure measure
The expanding novel medication endorsements and developing pharmaceutical industries is providing opportunity for hERG screening market. hERG screening is basically undertaken for medication disclosure measure. During the forecast period the expanding number of medications is foreseen to support the interest for hERG screening.
According to a report by Centre for Drug Evaluation and Research, in 2016, 25 medications were got novel medication endorsement and 59 in 2018 after HERG screening. Thus this fosters the hERG screening sector leading to better opportunity for market expansion.
Furthermore, the growing challenges in developing a pharmaceutical or biotechnological product based on application is helping to construct opportunities. Hence, this aids in revenue generation of hERG screening market.
Increase in manufacturing potential provides fruitful opportunities
The global manufacturers involved in manufacturing of the products required for hERG screening have improved their product manufacturing strategies in North America followed by Europe and APAC. This increase in manufacturing potential is due to the rise in number of cardiovascular diseases in the region, so as to survive in the market climate. In addition to this, the development of healthcare infrastructure, supporting policies by government and obese population suffering from heart illnesses, is expected to provide fruitful opportunities in the future.
Advanced Technologies in hERG Screening
One of the major concern during drug development leading to development termination and market withdrawal is Cardiotoxicity. 28-42% of all preclinical lead compounds are discontinued because of hERG ion channel inhibition. When there is drug induced interference of hERG it leads to QT interval prolongation, which results in fatal ventricular tachyarrhythmia called Torsade de Pointes (TdP). Due to these factors hERG assessment has become top priority by regulatory authorities before IND submission. There are some of the recently developed assays to determine test compounds causing hERG inhibition.
- Microelectrode Array
Microelectrode array can analyze changes in all major ion channels, ECG-like recordings. The tissue model is comprised of highly IPSC-induced human cardio-myocyte, cardiac endothelial and fibroblast cells. The model mimics spontaneous beating heart with electrophysiological and biochemical responses. This novel in vitro system has revealed a promising method for efficient phenotypic screening in drug discovery.
- Fluorescence Polarization Assay
A highly reproducible combination of specific fluorescence tracer and membrane preparation, this assay is able to detect certain substance with binding affinity to hERG channel quickly. Highly automatic control, precise protocols and rigorous validation process can ensure panoptic and reliable conclusions on hERG risks.
Impact of Macroeconomic Factors
Due to Covid 19 the market for hERG screening dropped significantly in initial times, as there was supply chain disruptions, travel restrictions and social distancing issues. But the manufacturers, developers and service providers contemplated the opportunity during difficult times and adopted various strategies to stabilize their profit.
As there were no diagnostic kits during early phase of Covid 19 to detect disease in patients. This unavailability of Covid 19 diagnostic kits gave lucrative opportunities for diagnostic manufacturers to introduce their Covid 19 diagnostic kits. Introduction of these kits into local as well as global markets was done by many leading key players as well as start-ups from various countries. Thus these market players capitalized the opportunity from demand for Covid 19 diagnostic kits, maintaining the economy in such a crisis.
- In 2020, Bio-Merieux, a French based biotech company gained an emergency use authorization for its VIDAS SARS-COV-2 test, which is an immunoassay used to detect IgG and IgM antibodies, to detect coronavirus. This led the company to generate a revenue of $3.6 billion for 2020.
- Bio-Rad, an American based company developed diagnostic kits for individuals suspected with Covid 19, generating a revenue of $2.5 billion in 2020.
Regional Analysis
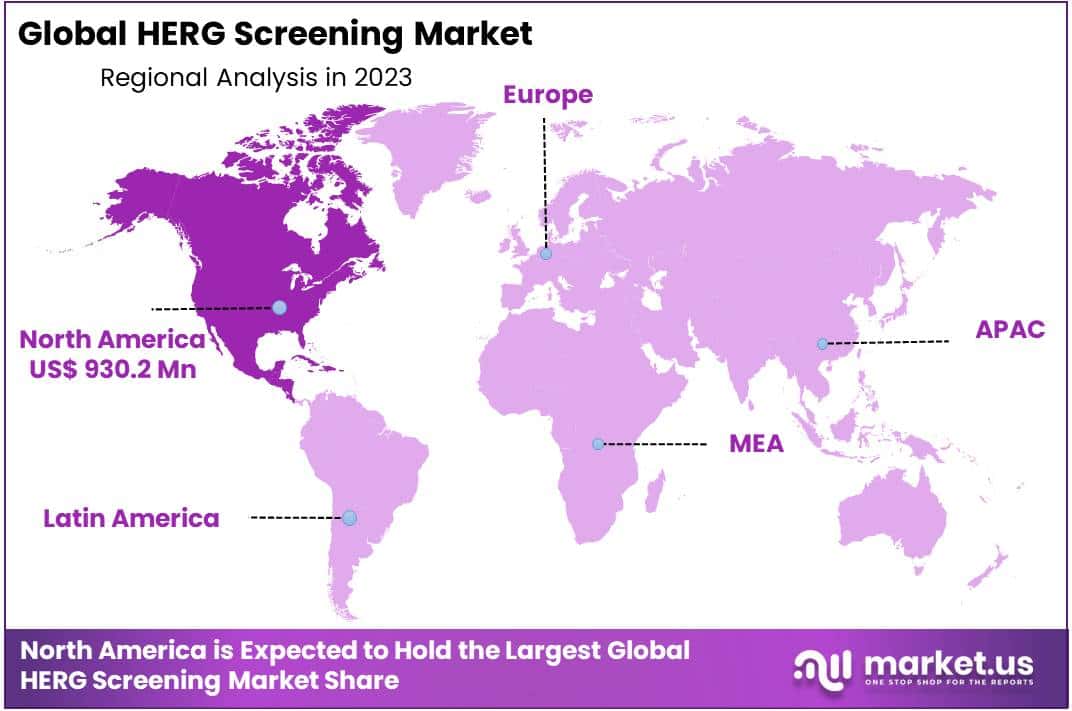
North America to witness significant growth during the forecast period
Contributing to the market growth, North America proves to be the largest market share holder of hERG screening, dominating the regional segment by 48.3% in 2023. The reason for this lucrative growth is the increasing number of myocardial infarction among citizens in United States, according to American College of Cardiology. The amassed number of heart illnesses among people has to undergo hERG screening and thus lead to its market expansion.
Besides, Europe also provide a healthy support to the market growth of hERG screening. U.K has the fastest growing market in the European region. Due to notable portion of people having chronic heart conditions and quick rise of inflammatory disorders, Asia Pacific region is expected to show significant market growth during the forecast period (2024-2033).
Key Regions and Countries
- North America
- The US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia & CIS
- Rest of Europe
- APAC
- China
- Japan
- South Korea
- India
- ASEAN
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- UAE
- Rest of MEA
Market Players Analysis
There are various companies that manufacture products or launch new techniques for hERG screening such as AVIVA Biosciences, Axxam, ABCAM plc, AstraZeneca plc, Aureus Sciences, Caliper Discovery Alliances and services, Charles River Laboratories, Creative Bio array, Cerep, ChanTest, Cyprotex Limited, Eurofins Scientific, etc.
In order to expand the footprints into the market these companies develop various strategies. These market developments include new product launches, contractual agreements, high investments, unifications and acquisitions, alliance with other organizations. Cost effective products is one of the eye catching tactic to expand and survive in rising market climate.
Key Market Players
- AVIVA Biosciences
- Axxam
- ABCAM plc
- AstraZeneca plc
- Aureus Sciences
- Caliper Discovery Alliances and services
- Charles River Laboratories
- Creative Bio array
- Cerep
- ChanTest
- Cyprotex limited
- Eurofins Scientific
Recent Developments
- In Aug 2023: Metrion Biosciences Limited is a specialist ion channel contract research and drug discovery company. The company is now a member of UK GLP (Good Laboratory Practice) compliance monitoring program due to which is now able to offer GLP compliant hERG ion channel screening facility to the life science community globally. The company now incorporates the new GLP hERG assay in addition to High throughput screening (HTS). This technology is with automated electrophysiology and fluorescence format, a wide ion channel cell biology potential, broadly helping in neuroscience drug discovery research.
- In March 2020: A collaboration was formed between Charles River Laboratories and Deciphex, a trailblazer in preclinical digital pathology software as a service. To aid hasten pathology analytics, the organization will collaborate to co-develop deep learning enabled technologies. Facilitating the patholytic preclinical solution to clients, Charles River will serve as the sole contract research organization.
Report Scope
Report Features Description Market Value (2023) USD 1,938.0 Million Forecast Revenue (2033) USD 6,578.7 Million CAGR (2024-2033) 13.04% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Product Type(Gene KCNH2, mutant KCNH2), by ION channel(voltage gated, ligand gated, mechanosensitive, transient receptor potential), by application (antiarrhythmic, antipsychotic, antibiotic, antimalarial, antihistamine) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape AVIVA Biosciences, Axxam, ABCAM plc, AstraZeneca plc, Aureus Sciences, Caliper Discovery Alliances and services, Charles River Laboratories, Creative Bio array, Cerep, ChanTest, Cyprotex Limited, Eurofins Scientific. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is HERG screening?HERG screening refers to the process of assessing the Human Ether-a-go-go-Related Gene (HERG) for abnormalities or mutations. This gene is associated with cardiac ion channels and is crucial in evaluating potential risks of drug-induced arrhythmias, particularly torsades de pointes.
How big is the HERG Screening Market?The global HERG Screening Market size was estimated at USD 1,938.0 Million in 2023 and is expected to reach USD 6578.7 Million in 2033.
What is the HERG ScreeningMarket growth?The global HERG Screening Market is expected to grow at a compound annual growth rate of 13.04%. From 2024 To 2033
Who are the key companies/players in the HERG Screening Market?Some of the key players in the HERG ScreeningMarkets are AVIVA Biosciences, Axxam, ABCAM plc, AstraZeneca plc, Aureus Sciences, Caliper Discovery Alliances and services, Charles River Laboratories, Creative Bio array, Cerep, ChanTest, Cyprotex Limited, Eurofins Scientific.
Why is HERG screening important?HERG screening plays a vital role in drug development and safety evaluation by identifying compounds that may prolong the QT interval, a risk factor for life-threatening arrhythmias. It helps pharmaceutical companies assess the cardiac safety profile of new drugs and mitigate the risk of adverse effects.
What are the key market drivers for HERG screening?The increasing focus on drug safety, stringent regulatory requirements, and growing demand for personalized medicine are key drivers fueling the growth of the HERG screening market. Additionally, advancements in technology, such as high-throughput screening methods and automated platforms, are enhancing efficiency and accuracy in HERG screening processes.
What are the future prospects for the HERG screening market?The HERG screening market is expected to witness robust growth, driven by increasing pharmaceutical research and development activities, rising awareness about drug-induced cardiac arrhythmias, and the expanding application of HERG screening in drug safety assessment. Technological advancements and collaborations between pharmaceutical companies and research institutions are likely to further propel market growth.

-
-
- AVIVA Biosciences
- Axxam
- ABCAM plc
- AstraZeneca plc
- Aureus Sciences
- Caliper Discovery Alliances and services
- Charles River Laboratories
- Creative Bio array
- Cerep
- ChanTest
- Cyprotex limited
- Eurofins Scientific










