Global Gynecological Dilators Market By Product Type (Silicone Dilators, Plastic Dilators, Metal Dilators and Glass Dilators), By Size (Medium Dilators, Small Dilators, Large Dilators and Customizable Size Dilators), By Application (Medical and Personal), By Distribution Channel (Hospitals & Clinics, Online Retailers, Medical Supply Stores and Pharmacies), By Consumer Type (Healthcare Professionals, Caregivers, Patients Undergoing Treatment and Individuals Seeking Personal Care Solutions), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Jan 2026
- Report ID: 173300
- Number of Pages: 359
- Format:
-
keyboard_arrow_up
Quick Navigation
- Report Overview
- Key Takeaways
- Product Type Analysis
- Size Analysis
- Application Analysis
- Distribution Channel Analysis
- Consumer Type Analysis
- Key Market Segments
- Drivers
- Restraints
- Opportunities
- Impact of Macroeconomic / Geopolitical Factors
- Latest Trends
- Regional Analysis
- Key Players Analysis
- Recent Developments
- Report Scope
Report Overview
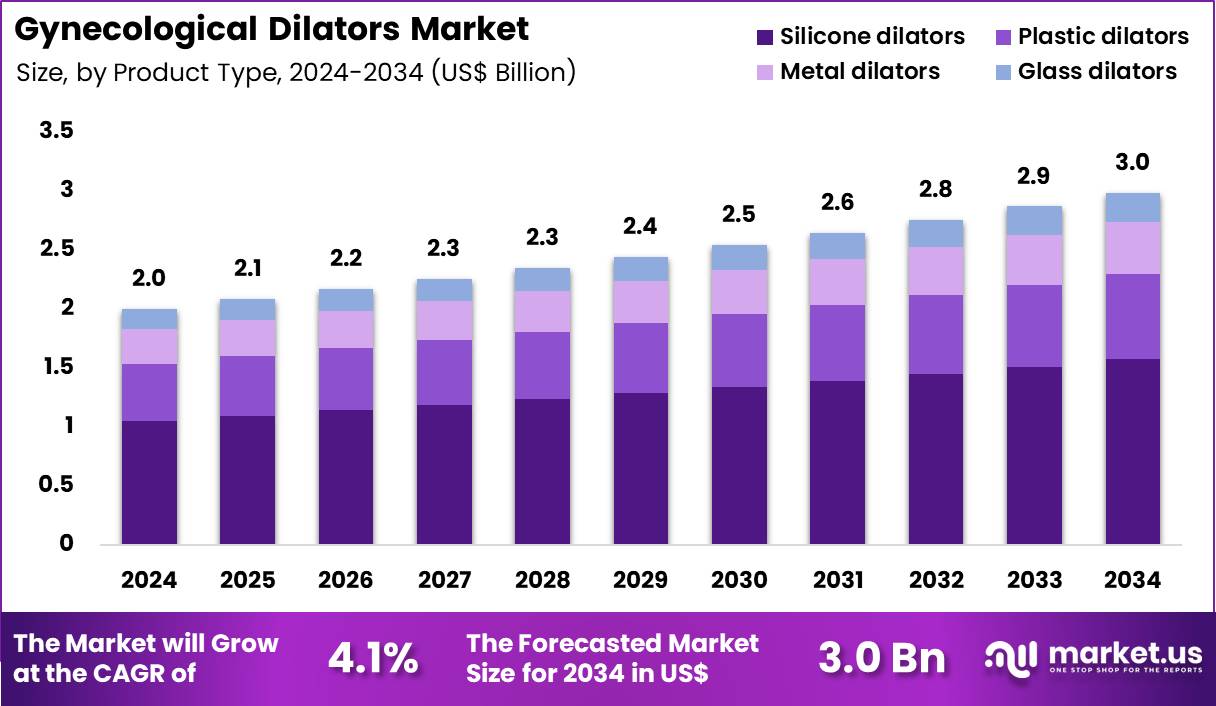
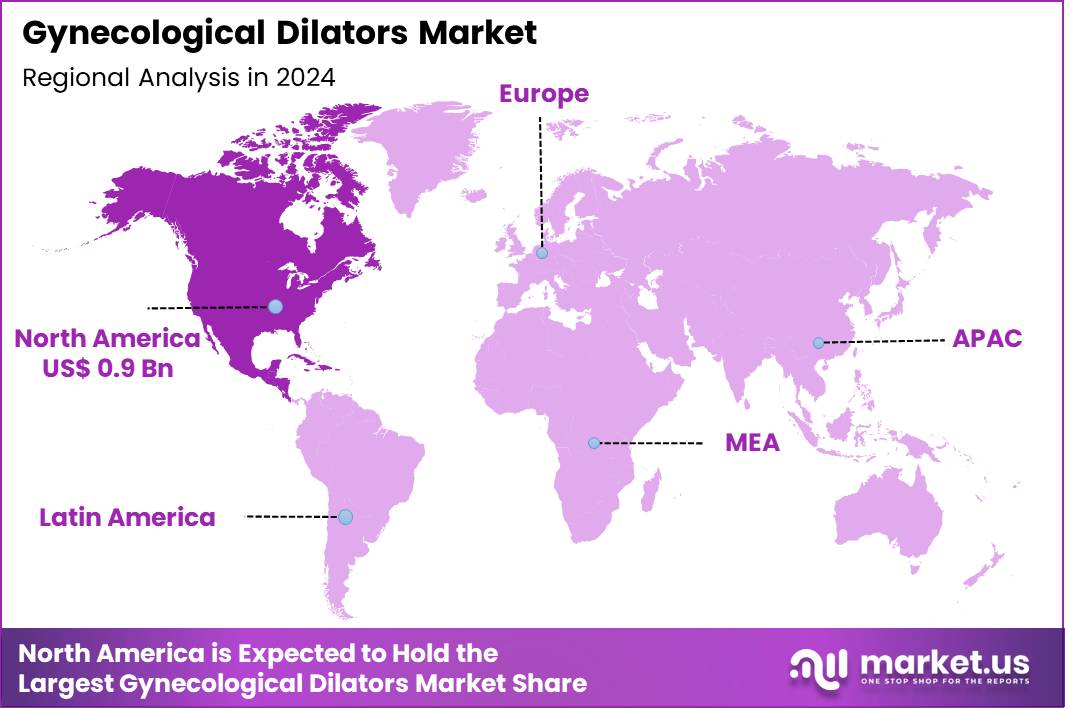
The Global Gynecological Dilators Market size is expected to be worth around US$ 3.0 Bllion by 2034 from US$ 2.0 Billion in 2024, growing at a CAGR of 4.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 42.8% share with a revenue of US$ 0.9 Billion.
Increasing prevalence of gynecological conditions and the emphasis on minimally invasive procedures drive demand for gynecological dilators that facilitate safe and effective cervical and vaginal access during diagnostic and therapeutic interventions. Obstetricians and gynecologists routinely employ cervical dilators to prepare the cervix for procedures such as hysteroscopy, dilation and curettage, and intrauterine device insertion, ensuring precise instrumentation without excessive trauma.
These devices support endometrial biopsy and aspiration by gradually widening the cervical os, enabling accurate sampling for pathology evaluation in abnormal uterine bleeding cases. Clinicians utilize vaginal dilators in reconstructive surgery to maintain or restore vaginal patency after procedures involving radiation therapy for gynecologic cancers. These tools also aid in the management of vaginal stenosis, promoting tissue expansion to alleviate discomfort in pelvic examinations and sexual function.
In January 2025, a leading gynecological device manufacturer completed the acquisition of a startup specializing in digital women’s health technologies. This move enables the integration of digital tools with conventional gynecological devices, supporting more connected care models and enhancing patient engagement, monitoring, and education.
Manufacturers pursue opportunities to advance osmotic and mechanical dilators that offer controlled expansion and reduced procedural time, expanding applications in fertility treatments such as embryo transfer and intrauterine insemination. Developers engineer disposable, single-use variants to minimize infection risks, broadening utility in outpatient settings for routine cervical ripening prior to labor induction.
These innovations facilitate therapeutic dilation in conditions like cervical stenosis, providing gentle progressive widening for improved patient tolerance during ongoing management. Opportunities emerge in integrating ergonomic designs and lubricated surfaces for enhanced comfort in pelvic floor therapy and post-brachytherapy rehabilitation.
Companies advance hybrid systems combining dilation with real-time imaging guidance, optimizing precision in complex diagnostic hysteroscopies and minimally invasive uterine surgeries. Firms invest in patient-specific sizing kits that support home-based therapy for vaginismus, improving long-term adherence in sexual health rehabilitation programs.
Industry leaders introduce silicone-based and polymer dilators with progressive sizing sets that promote gradual tissue adaptation, minimizing discomfort in postoperative vaginal reconstruction and gender-affirming surgery aftercare. Developers refine double-ended metal dilators for versatility in measuring and dilating during advanced hysteroscopic procedures.
Market participants prioritize antimicrobial coatings on reusable dilators, enhancing safety in high-volume clinical environments for repeated cervical preparations. Innovators incorporate flexible materials that reduce trauma in sensitive anatomies during brachytherapy preparation and chronic pelvic pain management.
Companies emphasize educational integration with digital platforms to guide proper dilation techniques in at-home vulvovaginal atrophy treatment. Ongoing advancements focus on compact, portable designs that support multidisciplinary care in urogynecology and oncology, elevating procedural outcomes across diverse gynecological applications.
Key Takeaways
- In 2024, the market generated a revenue of US$ 2.0 Billion, with a CAGR of 4.1%, and is expected to reach US$ 3.0 Billion by the year 2034.
- The product type segment is divided into silicone dilators, plastic dilators, metal dilators and glass dilators, with silicone dilators taking the lead in 2023 with a market share of 52.6%.
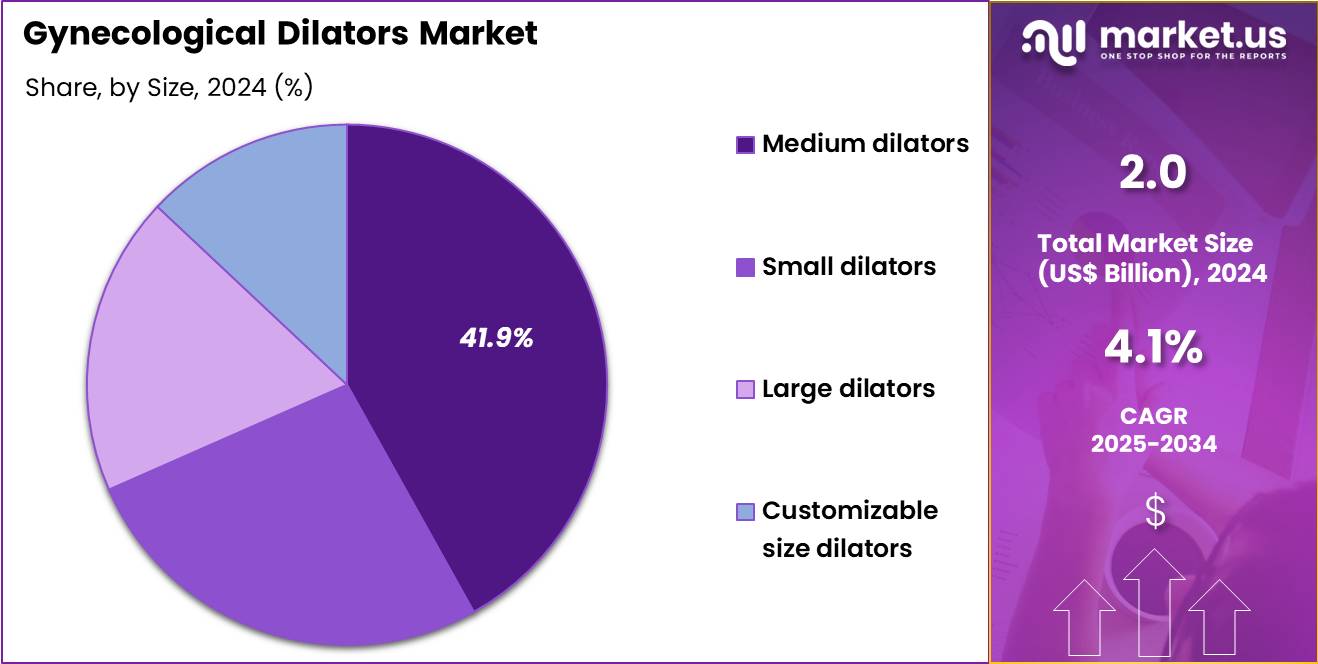
- Considering size, the market is divided into medium dilators, small dilators, large dilators and customizable size dilators. Among these, medium dilators held a significant share of 41.9%.
- Furthermore, concerning the application segment, the market is segregated into medical and personal. The medical sector stands out as the dominant player, holding the largest revenue share of 68.4% in the market.
- The distribution channel segment is segregated into hospitals & clinics, online retailers, medical supply stores and pharmacies, with the hospitals & clinics segment leading the market, holding a revenue share of 46.8%.
- Considering consumer type, the market is divided into healthcare professionals, caregivers, patients undergoing treatment and individuals seeking personal care solutions. Among these, healthcare professionals held a significant share of 44.7%.
- North America led the market by securing a market share of 42.8% in 2024.
Product Type Analysis
Silicone dilators lead the gynecological dilators market with a 52.6% growth share, driven by strong clinical preference for their soft texture and high biocompatibility. Healthcare professionals favor silicone for its comfort, flexibility, and low risk of irritation, which improves patient adherence during long-term therapy.
Medical-grade silicone supports strict safety and hygiene standards while allowing designs that balance stability with gentle tissue contact. These properties make silicone dilators well suited for post-surgical and post-radiation rehabilitation.
Easy sterilization, reusability, and low allergenic risk further support adoption in hospitals and clinics. Growing awareness of conditions such as vaginismus and vaginal stenosis, combined with patient trust in silicone materials, is expected to sustain segment leadership.
Size Analysis
Medium-sized dilators account for a 41.9% growth share, reflecting their role as the most commonly recommended size in clinical practice. Clinicians often select medium dilators as the starting point for therapy because they enable gradual dilation without excessive discomfort.
Their versatility suits a broad range of anatomical needs, simplifying prescribing and inventory management for hospitals and clinics. Medium dilators are widely used across both introductory and maintenance phases of treatment, supporting consistent patient compliance. Balanced rigidity and flexibility, along with lower risk of pain or injury, reinforce clinician confidence and continued preference for this size category.
Application Analysis
Medical use dominates the gynecological dilators market with a 68.4% growth share, supported by widespread integration into post-surgical, post-radiation, and congenital condition management. Dilators are embedded in standardized treatment pathways for vaginal stenosis, pelvic floor disorders, and cancer-related rehabilitation.
Rising survival rates in gynecological cancers are increasing long-term rehabilitation needs, strengthening medical demand. Evidence-based guidelines, physician supervision, and structured dosing protocols support consistent use. Medical applications also reduce the need for corrective surgeries, improving patient outcomes and quality of life, which sustains long-term segment dominance.
Distribution Channel Analysis
Hospitals and clinics represent the leading distribution channel with a 46.8% growth share, as most gynecological dilator therapy is initiated and supervised in institutional settings. Hospitals serve as primary diagnostic and treatment centers, while clinics provide follow-up care and therapy monitoring.
Centralized procurement ensures product quality and regulatory compliance, and bulk purchasing supports steady demand. Direct patient education, clinician-led guidance, and structured rehabilitation programs strengthen adoption. Institutional trust and standardized protocols continue to reinforce the dominance of hospitals and clinics in distribution.
Consumer Type Analysis
Healthcare professionals form the largest consumer group with a 44.7% growth share, reflecting their central role in prescribing and managing dilator therapy. Gynecologists, pelvic floor specialists, and rehabilitation teams determine device selection, size progression, and treatment duration. Professional oversight improves correct usage, safety, and patient adherence.
Clinician recommendations strongly influence procurement decisions within hospitals and clinics. Ongoing training, clinical documentation, and guideline-driven care further strengthen professional influence, supporting continued growth as specialized pelvic health services expand.
Key Market Segments
By Product Type
- Silicone dilators
- Plastic dilators
- Metal dilators
- Glass dilators
By Size
- Medium dilators
- Small dilators
- Large dilators
- Customizable size dilators
By Application
- Medical
- Personal
By Distribution Channel
- Hospitals & clinics
- Online retailers
- Medical supply stores
- Pharmacies
By Consumer Type
- Healthcare professionals
- Caregivers
- Patients undergoing treatment
- Individuals seeking personal care solutions
Drivers
Increasing number of abortion procedures is driving the market
The vaginal inserts market is significantly driven by the increasing number of abortion procedures, where dilators are essential for cervical preparation to ensure safe and effective access during surgical terminations. Healthcare providers rely on dilators to minimize risks such as cervical laceration and to facilitate outpatient procedures, enhancing patient safety. Regulatory bodies monitor abortion trends to inform guidelines on dilator use in reproductive health services.
Pharmaceutical companies develop dilators compatible with medication-induced abortions to support comprehensive care. Clinical protocols emphasize standardized dilation techniques to optimize outcomes in high-volume settings. Global health organizations advocate for access to safe abortion services, sustaining demand for reliable dilators.
Academic research on procedural safety contributes to innovations in dilator design for reduced discomfort. Patient rights movements promote availability of dilators in legal abortion frameworks. Economic factors highlight the cost-effectiveness of efficient dilation in ambulatory care. According to the Centers for Disease Control and Prevention’s Abortion Surveillance report for 2022, 625,978 abortions were reported from 49 reporting areas in the United States.
Restraints
Regulatory enforcement requiring prescription for dilators is restraining the market
The vaginal inserts market is restrained by regulatory enforcement requiring prescription for dilators, which limits over-the-counter availability and impacts consumer direct access. Manufacturers must comply with new classification rules, adding administrative burdens and delaying product distribution. Healthcare professionals face changes in prescribing practices, complicating routine use in physical therapy.
Regulatory agencies prioritize safety concerns, leading to stricter labeling and marketing restrictions. Clinical applications in pelvic health are affected, as non-prescription sales channels contract. Global variations in enforcement create inconsistencies for international suppliers. Academic associations advocate for clarity on the ruling to mitigate market disruption.
Patient access to self-care tools decreases, particularly for non-medical conditions like vaginal stenosis. Economic implications include reduced revenue from OTC segments for affected companies. According to the American Physical Therapy Association’s update published March 31, 2024, the FDA handed down an enforcement decision requiring prescription for vaginal dilators.
Opportunities
Expansion in contraceptive use requiring cervical dilation is creating growth opportunities
The vaginal inserts market presents growth opportunities through the expansion in contraceptive use, particularly intrauterine devices that often require cervical dilation for insertion. Healthcare initiatives promote long-acting reversible contraceptives, increasing the need for dilators in family planning clinics. Regulatory recommendations support safe IUD placement, encouraging innovation in dilator technologies for comfort.
Pharmaceutical collaborations focus on dilators compatible with hormonal and non-hormonal devices. Clinical research on contraceptive efficacy highlights the role of proper dilation in reducing expulsion rates. Global health programs target underserved populations, broadening market reach for dilator products. Academic studies explore dilation methods to improve acceptance of contraceptives. Patient education on reproductive options drives demand for minimally invasive insertion aids.
Economic benefits arise from preventing unintended pregnancies through effective contraceptive placement. According to the Centers for Disease Control and Prevention’s U.S. Selected Practice Recommendations for Contraceptive Use published August 8, 2024, updates address bleeding irregularities with levonorgestrel IUD use, reflecting ongoing focus on IUD-related procedures.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic factors actively drive growth in the Gynecological Dilators market as rising global GDP and increased healthcare investments expand demand for advanced medical procedures. However, persistent inflation pressures manufacturers to raise production costs, which squeezes profit margins and limits accessibility in emerging economies. Geopolitical tensions, including ongoing conflicts in Eastern Europe and the Middle East, disrupt global supply chains and delay raw material deliveries for dilator production.
On the positive side, companies diversify their sourcing strategies to mitigate these risks, fostering resilience through multi-regional partnerships. Current US tariffs on imported medical devices, ranging from 10% to 60% on goods from key suppliers like China, elevate import expenses and challenge international competitiveness.
Domestic firms benefit as these tariffs encourage local manufacturing investments and reduce reliance on foreign suppliers. Despite these hurdles, innovation in biodegradable and patient-friendly dilators propels market expansion. Overall, strategic adaptations position the sector for sustained growth and enhanced stability in the coming years.
Latest Trends
Adoption of 3D-printed customized vaginal dilators is a recent trend
In 2025, the vaginal inserts market has demonstrated a prominent trend toward the adoption of 3D-printed customized vaginal dilators, which offer personalized dimensions for treating conditions like vaginal stenosis post-surgery. Manufacturers utilize 3D printing to create molds tailored to individual anatomy, improving fit and comfort.
Healthcare providers integrate these dilators into rehabilitation protocols for enhanced patient adherence. Regulatory considerations for custom devices emphasize biocompatibility and sterilization. Clinical case reports document successful outcomes in maintaining vaginal function with printed dilators. Academic research explores materials for optimal durability in customized applications.
Global collaborations advance printing techniques for accessible production in clinical settings. Patient feedback guides iterative designs to reduce irritation during therapy. Ethical protocols ensure informed consent for personalized dilator use. According to a case report published by the National Institutes of Health on May 29, 2025, a 3D-printed vaginal mold effectively maintained vaginal caliber after reconstructive surgery.
Regional Analysis
North America is leading the Gynecological Dilators Market
In 2024, North America commanded a 42.8% share of the global gynecological dilators market, bolstered by rising demands for minimally invasive procedures in women’s health and advancements in ergonomic designs that enhance patient comfort during examinations and treatments. Obstetricians and gynecologists increasingly utilized disposable and graduated sets for hysteroscopy and intrauterine device insertions, driven by federal health initiatives promoting preventive care amid escalating rates of endometriosis and fibroids.
Regulatory approvals from bodies like the Food and Drug Administration expedited introductions of biocompatible materials resistant to breakage, supporting expanded applications in ambulatory surgical centers where procedural efficiency remains paramount. Demographic shifts toward delayed childbearing amplified needs for cervical dilation tools in fertility assessments, while educational campaigns empowered patients to seek early interventions for pelvic pain syndromes.
Hospital networks invested in sterile, single-use variants to comply with infection control protocols, minimizing cross-contamination risks post-pandemic. Pharmaceutical collaborations refined expandable balloon dilators for therapeutic abortions and postpartum management, optimizing outcomes in high-volume clinics. Supply optimizations ensured consistent availability of size-varied kits, aligning with diverse anatomical requirements.
The Centers for Disease Control and Prevention reported that a total of 613,383 abortions occurred in the United States in 2022, highlighting the sustained procedural volumes necessitating reliable dilation instruments.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Healthcare stakeholders forecast robust progression in gynecological dilation instruments across Asia Pacific throughout the forecast period, as intensifying reproductive health programs confront surging incidences of cervical and uterine conditions amid rapid population growth. Specialists incorporate graduated plastic tools into routine colposcopies, tailoring interventions to address disparities in access for underserved ethnic groups.
National authorities subsidize procurement of reusable metal sets for public hospitals, equipping them to handle escalating demands from maternal health drives in tropical regions prone to infections. Biotech innovators customize flexible silicone variants, optimizing safety for adolescent gynecology amid nutritional deficiencies that exacerbate structural anomalies.
Cross-border consortia validate expandable designs through multicenter studies, enhancing precision in minimally invasive therapies for infertility treatments. Pharmaceutical firms adapt antimicrobial-coated products, countering microbial resistance in densely populated urban centers.
Community outreach teams educate midwives on proper application techniques, extending utility to remote villages grappling with limited surgical resources. A plenary report from the Asian Society of Gynecologic Oncology documented 397,000 newly diagnosed cervical cancer cases in Asia during 2022, underscoring the imperative for advanced procedural aids in diagnostic and therapeutic contexts.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the Gynecological Dilators market drive growth by refining device ergonomics, material biocompatibility, and graduated sizing to improve patient comfort and procedural efficiency across diagnostic and therapeutic use cases. Companies expand adoption through close engagement with gynecologists and pelvic-health specialists, embedding dilators into standardized care pathways for cervical procedures and post-treatment rehabilitation.
Commercial strategies emphasize portfolio breadth, offering reusable and single-use options that align with infection-control policies and varied clinical settings. Innovation priorities include softer polymers, modular sets, and compatibility with minimally invasive workflows that reduce trauma and recovery time.
Market expansion targets regions strengthening women’s health infrastructure and outpatient gynecology services. CooperSurgical exemplifies leadership with a focused women’s health portfolio, global manufacturing and distribution capabilities, and strong clinician relationships that support consistent uptake of gynecological devices worldwide.
Top Key Players
- Velvi
- Surgical Holdings UK
- Stingray Surgical Products
- STERIS Instrument
- Soul Source
- Sklar Corp
- Pelican Feminine Healthcare
- Panpac Medical
- Olympus
- Medgyn Products
Recent Developments
- In March 2025, a major medical device company introduced a new range of gynecological dilators developed with a focus on improved comfort and simpler handling for patients and clinicians. The launch reflects efforts to broaden product offerings within gynecological care and strengthen positioning in a competitive women’s health device market.
- In February 2025, a well-established healthcare organization announced a collaboration with a research institute to jointly develop next-generation gynecological treatment solutions, including advanced dilator designs. The partnership is intended to shorten development timelines and support the translation of research innovations into clinically effective products.
Report Scope
Report Features Description Market Value (2024) US$ 2.0 Billion Forecast Revenue (2034) US$ 3.0 Billion CAGR (2025-2034) 4.1% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Silicone Dilators, Plastic Dilators, Metal Dilators and Glass Dilators), By Size (Medium Dilators, Small Dilators, Large Dilators and Customizable Size Dilators), By Application (Medical and Personal), By Distribution Channel (Hospitals & Clinics, Online Retailers, Medical Supply Stores and Pharmacies), By Consumer Type (Healthcare Professionals, Caregivers, Patients Undergoing Treatment and Individuals Seeking Personal Care Solutions) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Velvi, Surgical Holdings UK, Stingray Surgical Products, STERIS Instrument, Soul Source, Sklar Corp, Pelican Feminine Healthcare, Panpac Medical, Olympus, Medgyn Products. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Gynecological Dilators MarketPublished date: Jan 2026add_shopping_cartBuy Now get_appDownload Sample
Gynecological Dilators MarketPublished date: Jan 2026add_shopping_cartBuy Now get_appDownload Sample -
-
- Velvi
- Surgical Holdings UK
- Stingray Surgical Products
- STERIS Instrument
- Soul Source
- Sklar Corp
- Pelican Feminine Healthcare
- Panpac Medical
- Olympus
- Medgyn Products










