Global Transdermal Drug Delivery Systems Market By Technology (Iontophoresis, Electroporation, Radio Frequency, Ultrasound and Others), By Application (Pain Management, Cardiovascular, Gastrointestinal, Cancer and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Jan 2026
- Report ID: 175142
- Number of Pages: 207
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
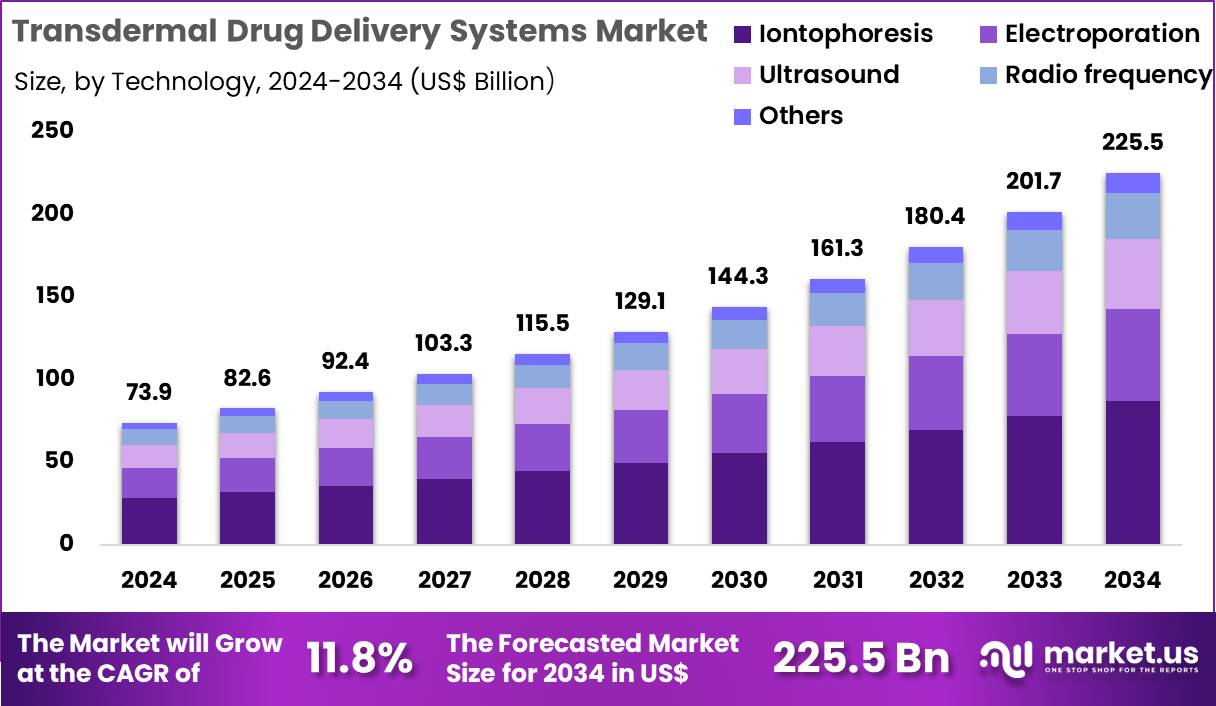
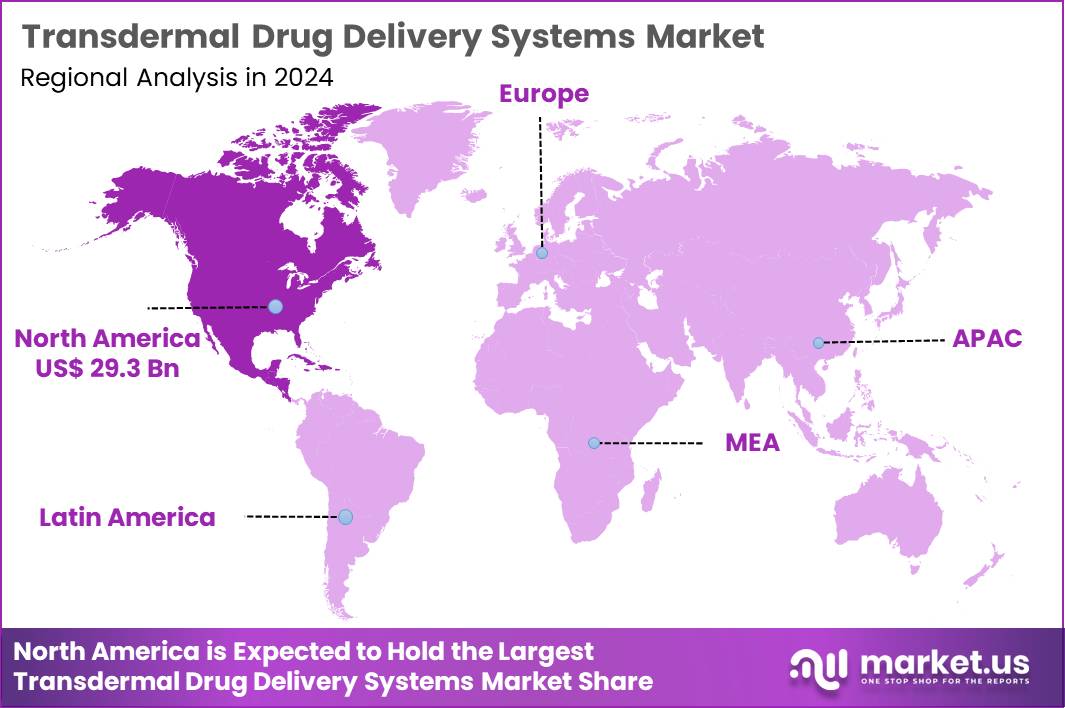
Global Transdermal Drug Delivery Systems Market size is expected to be worth around US$ 225.5 Billion by 2034 from US$ 73.9 Billion in 2024, growing at a CAGR of 11.8% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.7% share with a revenue of US$ 29.3 Billion.
Increasing patient preference for non-invasive and sustained-release therapies drives the transdermal drug delivery systems market as pharmaceutical companies innovate patches that bypass gastrointestinal degradation and improve compliance. Clinicians increasingly prescribe fentanyl transdermal patches for chronic pain management in cancer and arthritis patients, delivering consistent opioid levels over days to minimize breakthrough discomfort.
These systems support hormone replacement therapy through estradiol patches, regulating menopausal symptoms like hot flashes and bone density loss with steady dermal absorption. Dermatologists utilize nicotine replacement patches for smoking cessation, providing controlled nicotine doses to curb withdrawal symptoms and promote long-term abstinence.
Transdermal scopolamine applications address motion sickness in travelers and nausea in postoperative care, offering prolonged antiemetic effects without oral intake. Neurologists apply rivastigmine patches to manage Alzheimer’s symptoms, ensuring gradual acetylcholine esterase inhibition that enhances cognitive function over extended periods.
Manufacturers seize opportunities to integrate microneedle arrays into transdermal systems, enabling delivery of larger molecules like vaccines and biologics for broader applications in immunotherapy and infectious disease prevention. Developers advance iontophoretic patches that use mild electrical currents to drive drugs through skin barriers, expanding utility in localized pain relief and anti-inflammatory treatments.
These innovations facilitate wearable smart patches with embedded sensors that monitor adherence and adjust dosing in real time for personalized chronic disease management. Opportunities emerge in sustainable, biodegradable materials that reduce environmental impact while maintaining efficacy in long-wear hormone and analgesic therapies.
Companies invest in combination patches that co-deliver multiple actives, optimizing outcomes in complex conditions like hypertension or migraine prophylaxis. Recent trends emphasize digital connectivity in transdermal devices, linking to mobile apps for data tracking and telemedicine integration, elevating patient engagement across diverse therapeutic areas.
Key Takeaways
- In 2024, the market generated a revenue of US$ 73.9 Billion, with a CAGR of 11.8%, and is expected to reach US$ 225.5 Billion by the year 2034.
- The technology segment is divided into iontophoresis, electroporation, radio frequency, ultrasound and others, with iontophoresis taking the lead with a market share of 38.6%.
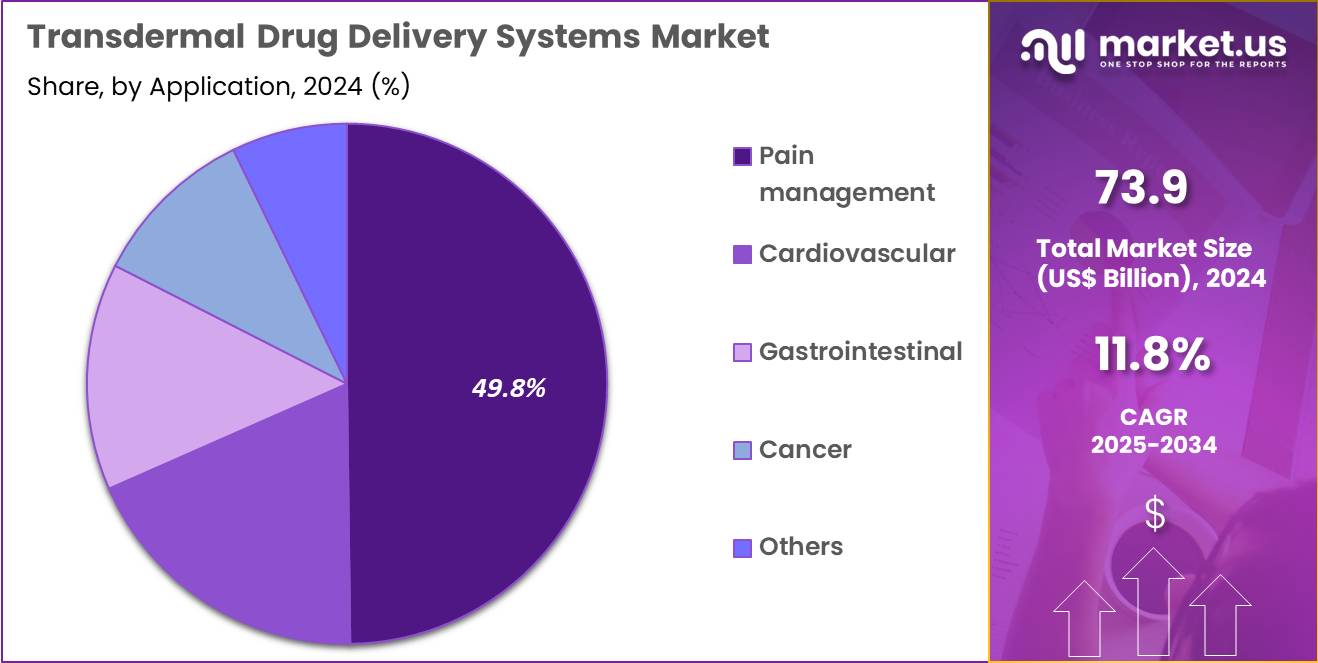
- Considering application, the market is divided into pain management, cardiovascular, gastrointestinal, cancer and others. Among these, pain management held a significant share of 49.8%.
- North America led the market by securing a market share of 39.7%.
Technology Analysis
Iontophoresis contributed 38.6% of growth within technology and remains the most adopted approach in the transdermal drug delivery systems market due to its controlled, needle-free delivery mechanism. Clinicians value iontophoresis because it uses a mild electrical current to drive charged drug molecules across the skin with predictable dosing. This level of control supports safer delivery compared with many passive patches, especially when therapy requires timed release or rapid onset.
Hospitals and specialty clinics also prefer iontophoresis for localized treatment, since the method reduces systemic exposure and improves tolerability in sensitive patients. Device manufacturers continue to improve wearable form factors, which increases practicality for ambulatory use and short outpatient procedures. Growing acceptance of non-invasive delivery strengthens demand in patients who avoid injections due to discomfort or adherence issues.
Iontophoresis also supports delivery of molecules that struggle to cross the skin barrier using conventional patch designs. Training requirements remain manageable, which supports adoption in physiotherapy, sports medicine, and pain clinics. Better battery efficiency and compact electronics reduce device size and improve patient comfort. Integration of dose control features strengthens clinician confidence in repeat usage.
The technology aligns well with home-based care trends because patients follow guided usage schedules without complex administration skills. Product developers also prioritize iontophoresis for differentiated drug-device combinations, supporting new launches and pipeline expansion. Reduced needle-stick risk improves safety in clinical environments with high patient turnover.
Shorter recovery time compared with invasive administration supports patient preference. Iontophoresis platforms also allow therapy customization by adjusting current intensity and treatment duration. Health systems increasingly emphasize adherence and convenience, and iontophoresis supports both objectives through structured delivery. The segment is anticipated to retain dominance due to dosing precision, patient comfort, and strong fit with drug-device innovation strategies.
Application Analysis
Pain management accounted for 49.8% of growth within application and leads the transdermal drug delivery systems market because pain therapy requires sustained, convenient, and patient-friendly dosing options. High prevalence of chronic musculoskeletal pain, neuropathic pain, and post-operative discomfort increases demand for alternatives to oral analgesics.
Clinicians prefer transdermal delivery in many cases because it avoids first-pass metabolism and supports steadier drug levels over time. Patient adherence improves when therapy eliminates frequent pill intake, especially in long-term pain conditions. Pain clinics adopt transdermal systems for localized drug delivery, which supports better control with fewer systemic effects.
Growing focus on opioid-sparing strategies increases interest in non-invasive modalities that deliver non-opioid agents through the skin. Sports injuries and rehabilitation settings also raise demand for transdermal pain options due to ease of use during recovery periods.
Elderly patients managing arthritis and chronic back pain strengthen usage because they often require long-duration therapy with minimal gastrointestinal burden. Transdermal approaches support functional recovery by enabling continuous pain relief during daily activity. Product improvements in adhesives and skin tolerability reduce irritation, which increases repeat use.
Combination pain therapies also benefit from controlled transdermal release profiles. Employers and insurers increasingly support pain management approaches that reduce hospital visits, which strengthens demand for at-home options. Digital health integration and reminders improve adherence in chronic pain patients.
Increased outpatient procedures drive short-term pain management needs that fit transdermal delivery. The segment is projected to remain dominant due to large patient volume, long treatment duration, and strong demand for convenient non-invasive pain control.
Key Market Segments
By Technology
- Iontophoresis
- Electroporation
- Radio Frequency
- Ultrasound
- Others
By Application
- Pain Management
- Cardiovascular
- Gastrointestinal
- Cancer
- Others
Drivers
The rising prevalence of chronic conditions is driving the market
The escalating incidence of chronic diseases, particularly those requiring long-term medication adherence, is a primary driver for the transdermal drug delivery systems market. Conditions such as Parkinson’s disease and chronic pain require steady-state plasma concentrations that transdermal patches provide more effectively than fluctuating oral doses. Major pharmaceutical players have observed significant revenue contributions from their transdermal portfolios to meet this clinical demand.
For instance, Viatris reported that its total net sales for the full year 2023 reached US$ 15.4 billion, with its “Brands” segment, which includes various transdermal products, contributing US$ 9.8 billion. Furthermore, Viatris reported that its new product revenues, including specialized delivery systems, totaled US$ 582 million in 2024.
The shift toward non-invasive delivery is also supported by the increasing volume of geriatric patients who often face dysphagia or difficulty swallowing pills. According to the National Cancer Institute, there were an estimated 2,001,140 new cancer cases in the US in 2024, many of which utilize transdermal fentanyl for chronic pain management.
Government data highlights that the stability of drug release in patches reduces the risk of gastrointestinal side effects commonly associated with oral analgesics. As healthcare providers focus on improving patient compliance, the adoption of transdermal systems for hormonal and cardiovascular therapies continues to rise. Consequently, the integration of these systems into standard chronic care protocols remains a fundamental driver for market growth.
Restraints
Rigorous regulatory requirements and technical barriers are restraining the market
The transdermal drug delivery systems market is significantly restrained by stringent regulatory oversight and the inherent difficulty of formulating drugs that can penetrate the skin barrier. Regulatory bodies like the FDA require extensive clinical data to prove bioequivalence and adhesive safety, which increases the time-to-market for new generic patches.
In 2024 and 2025, the implementation of updated FDA guidance on the quality and performance of transdermal systems has raised the bar for manufacturing standards. These requirements can lead to financial setbacks for major players; for example, Viatris noted in its 2024 financial results that divestiture-adjusted operational revenues were impacted by various regulatory and market shifts.
The physical properties of the skin’s stratum corneum also limit the types of molecules that can be delivered, effectively excluding most large-molecule biologics. Furthermore, skin irritation and contact dermatitis remain common adverse effects that lead to high patient discontinuation rates.
Manufacturing complexities also add to the burden, as maintaining a precise drug-in-adhesive matrix requires highly specialized equipment. These technical and economic hurdles often deter smaller pharmaceutical firms from entering the space due to high R&D costs. Despite the advantages of patches, these safety-related and financial barriers represent a substantial check on the speed of market penetration.
Opportunities
The development of microneedle technology is creating growth opportunities
Advancements in microneedle technology represent a transformative growth opportunity by expanding the range of drugs that can be delivered through the skin. Microneedles create microscopic channels in the stratum corneum, allowing for the delivery of high-molecular-weight drugs and vaccines that were previously limited to injections.
In 2023, Micron Biomedical received US$ 23.6 million in funding for the manufacturing scale-up of needle-free vaccine administration patches, highlighting the sector’s potential. Similarly, Vaxess Technologies secured approximately US$ 12 million in 2024 to advance its microarray patch platform for vaccines and GLP-1 delivery. This technology is particularly promising for global immunization programs, as it eliminates the need for cold-chain storage and specialized medical personnel for administration.
Research published by the NIH in 2025 indicates that microneedle-based systems are poised to target a multibillion-dollar segment of the biologics and vaccine markets. These systems also offer improved bioavailability and a faster onset of action compared to traditional passive patches.
As clinical trials for microneedle-delivered insulin and hormones progress, the technology is expected to gain significant traction in the outpatient setting. Manufacturers that invest in these next-generation delivery platforms are well-positioned to lead the transition toward painless, self-administered medicine.
Impact of Macroeconomic / Geopolitical Factors
Global economic progress channels resources into patient-centric drug delivery innovations, strengthening the transdermal drug delivery systems market as companies advance non-invasive patches for chronic conditions like pain management and hormone therapies. Leaders exploit demographic trends toward extended lifespans in key markets, which broadens applications and sustains steady revenue from improved compliance solutions.
Nonetheless, widespread inflation worldwide inflates costs for polymers, adhesives, and active ingredients, obliging producers to navigate tighter margins in price-sensitive segments. Geopolitical strains in raw material sourcing regions interrupt consistent flows of specialized chemicals, testing supply reliability for multinational developers.
Managers counter these disruptions by building diversified networks with dependable partners, which enhances stability and reveals opportunities for streamlined collaborations. Current US tariffs on imported pharmaceuticals and related components from primary suppliers impose added financial pressures, complicating entry for foreign-reliant systems targeting the American market.
Domestic innovators respond by intensifying local R&D and manufacturing efforts, which accelerates formulation breakthroughs and supports supply chain security. Emerging microneedle and smart patch technologies continually invigorate the sector, promising greater therapeutic precision and sustained market vitality worldwide.
Latest Trends
The integration of AI and digital monitoring is a recent trend
A prominent trend in 2024 and early 2025 is the integration of Artificial Intelligence (AI) and digital sensors into “smart” transdermal delivery systems. These advanced patches are designed not only to deliver medication but also to monitor physiological signals and adjust dosage in real-time.
In October 2024, the FDA granted 510(k) clearance for innovative transdermal systems that incorporate electronic components for controlled drug release. This digital transformation is further evidenced by the rise of wearable biosensors that communicate directly with smartphone applications to track patient adherence.
For instance, in January 2025, the FDA approved the Exelon Patch for Alzheimer’s disease, which features improved delivery mechanisms to ensure consistent rivastigmine levels. Companies are also using AI algorithms to optimize the design of skin permeation enhancers, significantly reducing the R&D timeline for new formulations.
Strategic collaborations, such as the 2024 partnership between 3M and specialized pharmaceutical firms, focus on combining adhesive expertise with digital health platforms. This shift toward “theranostic” deviceswhich combine therapy and diagnostics is becoming a cornerstone of personalized medicine. As the healthcare industry moves toward data-driven care, the role of transdermal systems as an interface for both delivery and monitoring is rapidly evolving.
Regional Analysis
North America is leading the Transdermal Drug Delivery Systems Market
North America commands a 39.7% share of the global transdermal drug delivery systems market, demonstrating sustained expansion in 2024 through heightened preference for non-invasive administration methods amid rising chronic disease prevalence. Advancements in patch technologies, including microneedle arrays and iontophoresis, have improved drug permeation and patient adherence for conditions such as pain management and neurological disorders.
Leading pharmaceutical entities in the United States have prioritized these systems to minimize gastrointestinal side effects associated with oral formulations. Regulatory pathways from the FDA have facilitated approvals of novel patches, enhancing commercial viability and market penetration. Collaborative initiatives between industry and academic institutions have accelerated innovation in sustained-release mechanisms tailored to geriatric populations.
Increased focus on opioid-sparing alternatives has driven adoption in pain therapeutics, addressing public health concerns over dependency. Robust healthcare infrastructure supports widespread clinical integration and reimbursement for these delivery platforms. In 2022, the FDA approved the Adlarity donepezil transdermal system for Alzheimer’s disease treatment, enabling consistent weekly dosing and reduced gastrointestinal adverse effects.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Manufacturers and research entities actively expand operations in the Asia Pacific region for advanced skin-based drug administration during the forecast period, capitalizing on surging demand for convenient therapies in populous markets. China, India, and Japan invest heavily in pharmaceutical innovation to combat growing incidences of diabetes, cardiovascular ailments, and pain-related conditions.
Local firms develop cost-effective patches and collaborate with global partners to localize production and reduce dependency on imports. Rising healthcare expenditures and policy emphasis on domestic biotechnology strengthen infrastructure for clinical trials and manufacturing. Researchers integrate nanotechnology to overcome skin barrier challenges, broadening applicability across therapeutic categories.
Pharmaceutical companies prioritize these platforms in drug pipelines to improve compliance among aging demographics. Government initiatives promote research in novel delivery technologies, fostering ecosystem growth. In 2023, India’s government launched the Promotion of Research and Innovation in Pharma MedTech (PRIP) scheme to support biotechnology and medtech advancements, including innovative drug delivery approaches.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the Transdermal Drug Delivery Systems market drive growth by advancing patch technologies that deliver consistent dosing, reduce gastrointestinal side effects, and improve adherence in chronic therapies. Companies expand adoption by optimizing adhesives, wear comfort, and skin compatibility to support long-duration use across diverse patient populations.
Commercial strategies emphasize lifecycle management through reformulated patches and differentiated delivery profiles that extend product value beyond oral alternatives. Innovation priorities include microneedle-enhanced platforms and permeation technologies that broaden the range of drugs suitable for transdermal administration.
Market expansion targets regions with strong outpatient care models and rising demand for convenient, non-invasive drug delivery. Hisamitsu Pharmaceutical operates as a key participant with deep expertise in patch-based therapeutics, strong manufacturing capability, and global commercialization experience that supports sustained growth in transdermal therapy solutions.
Top Key Players
- 3M Company
- Hisamitsu Pharmaceutical Co., Inc.
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- UCB S.A.
- Kindeva Drug Delivery L.P.
- LTS Lohmann Therapie Systeme AG
- Luye Pharma Group Ltd.
- Noven Pharmaceuticals, Inc.
Recent Developments
- In 2024, Hisamitsu Pharmaceutical continued to demonstrate strong momentum in the transdermal therapeutics segment, supported by solid performance of its Salonpas franchise and prescription patch portfolio. The company has been prioritizing expansion in the US market while increasing investment in matrix type transdermal patches that enable more uniform and controlled drug release. These formulation advances support sustained therapeutic effectiveness in pain management and reinforce long term adoption of patch based delivery systems.
- In 2024, Viatris generated total revenue of US$ 14.7 billion, with transdermal therapies forming an important part of its diversified product base. The company reported US$ 582 million in revenue from newly launched products during the year, driven in part by complex generics such as transdermal systems for hormone therapy and pain relief. By expanding access to lower cost alternatives to branded patches, Viatris continues to use advanced transdermal technologies as a key contributor to portfolio growth and market competitiveness.
Report Scope
Report Features Description Market Value (2024) US$ 73.9 Billion Forecast Revenue (2034) US$ 225.5 Billion CAGR (2025-2034) 11.8% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Technology (Iontophoresis, Electroporation, Radio Frequency, Ultrasound and Others), By Application (Pain Management, Cardiovascular, Gastrointestinal, Cancer and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape 3M Company, Hisamitsu Pharmaceutical Co., Inc., Novartis AG, Teva Pharmaceutical Industries Ltd., Viatris Inc., UCB S.A., Kindeva Drug Delivery L.P., LTS Lohmann Therapie Systeme AG, Luye Pharma Group Ltd., Noven Pharmaceuticals, Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Transdermal Drug Delivery Systems MarketPublished date: Jan 2026add_shopping_cartBuy Now get_appDownload Sample
Transdermal Drug Delivery Systems MarketPublished date: Jan 2026add_shopping_cartBuy Now get_appDownload Sample -
-
- 3M Company
- Hisamitsu Pharmaceutical Co., Inc.
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- UCB S.A.
- Kindeva Drug Delivery L.P.
- LTS Lohmann Therapie Systeme AG
- Luye Pharma Group Ltd.
- Noven Pharmaceuticals, Inc.










