Global Antimicrobial Peptides Market Analysis By Type (Cationic Peptides, Linear Peptides, Cyclic Peptides, Alpha-Helical Peptides, Beta-Sheet Peptides, Other Peptides), By Source (Natural Sources, Synthetic Peptides, Recombinant Peptides, Engineered Peptides), By Application (Healthcare & Therapeutics, Agriculture & Animal Health, Cosmetics & Personal Care, Food & Beverages, Veterinary Medicine), By Route of Administration (Topical, Oral, Injectables, Inhalation, Others), By End-User (Pharmaceuticals, Biotechnology, Veterinary Clinics, Others) By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Oct 2025
- Report ID: 163297
- Number of Pages: 229
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
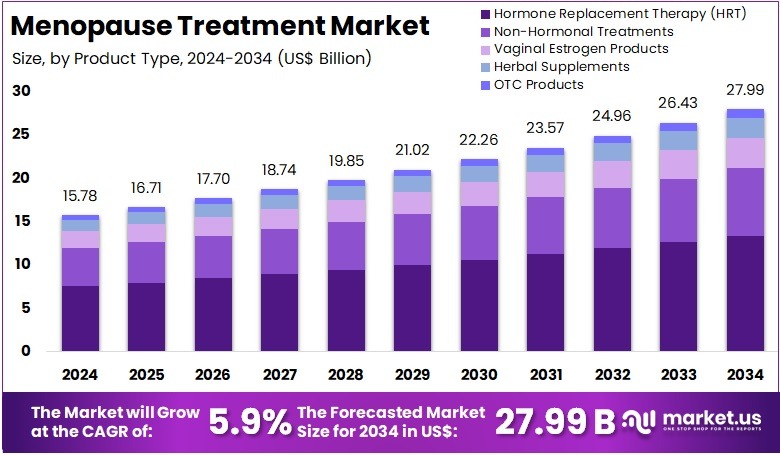
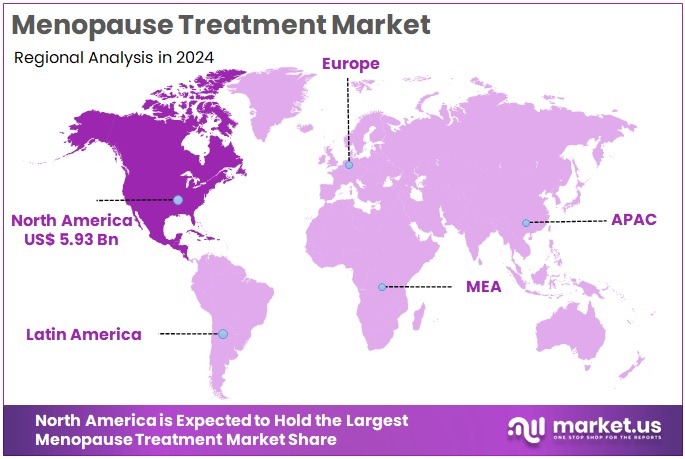
The Global Antimicrobial Peptides Market size is expected to be worth around US$ 776.5 Billion by 2034, from US$ 245.6 Billion in 2024, growing at a CAGR of 12.2% during the forecast period from 2025 to 2034. North America held a dominant market position, capturing more than a 39.7% share and holds US$ 97.5 Million market value for the year.
Antimicrobial Peptides (AMPs) are short protein molecules that work as natural defense agents against harmful pathogens. They are produced by humans, animals, plants, and bacteria. Their role is to destroy or inhibit the growth of bacteria, viruses, fungi, and parasites. AMPs are gaining attention because traditional antibiotics are losing effectiveness. Their broad-spectrum action and low resistance development make them strong candidates in next-generation antimicrobial solutions for medical, food, and personal care uses.
Market growth is strongly linked to rising antimicrobial resistance (AMR). The World Health Organization (WHO) estimates that 1.27 million deaths were directly caused by bacterial AMR in 2019, while 4.95 million deaths were associated with it. WHO has listed AMR among the top global health threats. For example, in the United States, the CDC reports more than 2.8 million resistant infections and over 35,000 deaths every year. When *Clostridioides difficile* is included, the burden rises to above 3 million infections and 48,000 deaths, creating steady demand for new treatment options like AMPs.
A study by WHO indicates growing supply-side momentum. As of 31 December 2023, 97 antibacterial agents were in clinical development. Among them, 40 candidates represent non-traditional approaches, including peptide-based solutions. However, only 15 products reached Phase 2/3 or Phase 3, which shows significant room for innovation and investment. This demonstrates that AMPs are positioned as an expanding early-stage opportunity within the global anti-infective pipeline.
Supportive funding and procurement policies also enhance market prospects. For instance, the U.S. Biomedical Advanced Research and Development Authority (BARDA) accepts proposals specifically for novel antibacterial countermeasures. Its Broad Agency Announcement highlights non-traditional mechanisms that outperform current treatments. This reduces financial risk for developers. In addition, hospitals are facing longer stays and higher costs due to resistance, which increases the need for fast-acting peptide-based therapeutics.
Regulatory progress is strengthening the pathway to approvals. In Europe, the European Medicines Agency has provided clear guidelines for antibacterial clinical trials. In the United States, the FDA’s Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) allows smaller and faster trials for serious infections. For example, the approval of Daptomycin in 2003 for complicated skin infections proves that peptide antibacterials can achieve regulatory success when supported by strong clinical data.
Scientific progress and better data systems reinforce market expansion. WHO reports that global AMR surveillance grew to 104 participating countries in 2023, compared with 25 in 2016. More than 23 million confirmed cases were analyzed to identify priority pathogens. This wider evidence base helps researchers target high-need areas where AMPs could deliver maximum benefit. Continuous advancements in biotechnology and peptide synthesis are expected to support commercial growth across pharmaceuticals, animal health, food preservation, and agricultural protection.
Key Takeaways
- The global antimicrobial peptides market is projected to reach US$ 776.5 Billion by 2034 from US$ 245.6 Billion in 2024, reflecting 12.2% CAGR growth.
- Cationic peptides led the type segment in 2024, accounting for over 34.4% market share due to their strong antimicrobial efficacy and widespread applications.
- Natural sources dominated the source segment in 2024, capturing more than 38.8% share, supported by rising preference for naturally derived therapeutic solutions.
- Healthcare and therapeutics applications held the largest contribution in 2024, securing over 44.2% share owing to increased disease-focused clinical utilization.
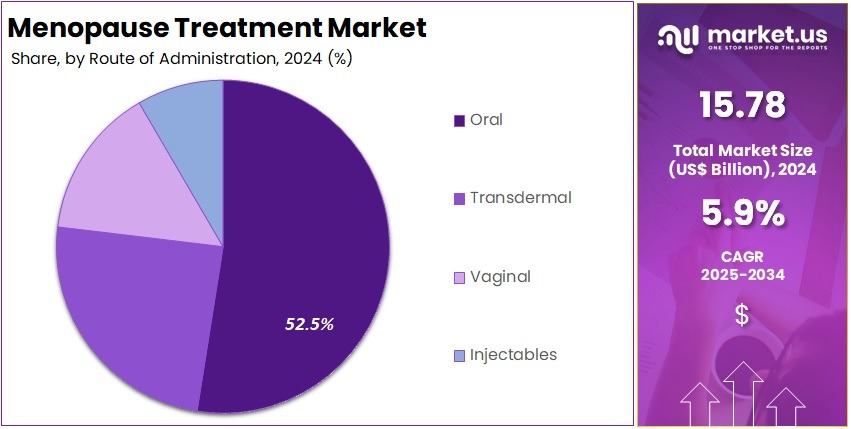
- Topical administration was the leading route in 2024, representing more than 34.5% share due to ease of use and patient compliance benefits.
- Pharmaceutical companies captured over 52.3% share in 2024 as the major end-users, driven by strong R&D investments in antimicrobial innovations.
- North America dominated regionally with a 39.7% share and US$ 97.5 Million value in 2024, supported by advanced healthcare infrastructure and funding.
Type Analysis
In 2024, the Cationic Peptides Section held a dominant market position in the Type Segment of the Antimicrobial Peptides Market, and captured more than a 34.4% share. These peptides bind with microbial membranes. Their broad activity supports high usage in infection control. Research spending has increased applications. Linear Peptides also gained a notable share. Their simple structures offer easy production. Antimicrobial response drives adoption in medicines. Analysts indicate steady growth for these peptide categories.
Cyclic Peptides are being viewed as a stable option. Their closed structures resist enzyme attack. This supports longer action in the body. Third-party reviews highlight attention from developers. Alpha-Helical Peptides also show selective toxicity toward microbes. Their effectiveness increases interest in therapeutic projects. New formulations are under progress. Market analysts state that both segments can benefit from rising antimicrobial resistance. Growth can be attributed to research support. Their roles in next generation drugs remain promising across global markets.
Beta-Sheet Peptides are at an early stage. They show bactericidal action that supports future demand. Experts view them as an upcoming area for investment. Clinical investigation continues. Other Peptides include hybrid and advanced formats. These candidates can improve performance against resistant pathogens. Their adoption will depend on trial success. Industry assessments predict faster expansion for these emerging categories. Stakeholders expect contributions to novel treatments. Continued innovation reinforces a positive outlook for every type segment.
Source Analysis
In 2024, the Natural Sources section held a dominant market position in the Source Segment of the Antimicrobial Peptides Market, and captured more than a 38.8% share. It has been observed that demand for natural antimicrobial peptides is rising. These products are valued for clean and safe therapeutic profiles. They are extracted from plants, microorganisms, and animals. Better biocompatibility and reduced toxicity support adoption. The growing focus on bio-based medicines has strengthened market preference. This trend is expected to continue.
It was reported that Synthetic Peptides represented a considerable portion of the market share. Synthetic methods support scalable and cost-efficient production. These peptides are modified to improve stability and performance. Adoption is growing due to active research efforts. Recombinant Peptides hold a smaller but promising presence. Biotechnology processes ensure higher purity and better yields. They address sourcing limitations faced by natural options. Healthcare applications continue rising. These advancements indicate sustained demand across both synthetic and recombinant segments.
Engineered Peptides are recognized as one of the fastest-growing segments. They are designed using computational and protein engineering tools. These peptides show strong targeting of pathogens. They also present high resistance against enzymatic breakdown. Their role in treating drug-resistant infections is gaining attention. Market analysts expect rapid expansion as precision therapies evolve. Overall, rising antimicrobial resistance is driving investments. Advancements across all peptide development methods are contributing to future growth of the global market.
Application Analysis
In 2024, the Healthcare & Therapeutics Section held a dominant market position in the Application Segment of Antimicrobial Peptides Market, and captured more than a 44.2% share. This leadership was supported by demand for new antimicrobial solutions. Antibiotic resistance challenges increased the need for these peptides. Their broad action against bacteria, fungi, and viruses improved medical outcomes. Hospitals adopted them for wound care and infection control. Researchers focused on their role in cancer and sepsis treatment. Investments in clinical studies strengthened the segment. Healthcare remained the highest revenue contributor.
Food preservation formed the next major application area during the same period. Natural preservatives gained preference due to clean-label trends. These peptides helped improve product safety and shelf life. Food producers used them in packaging films and coatings. Their benefits aligned with regulatory focus on reducing chemical additives. Growing awareness about foodborne pathogens increased adoption. This application area observed steady growth. It continued to expand across processed and ready-to-eat food categories.
Agriculture and personal care applications showed rising potential. Farmers used antimicrobial peptides as safe bio-pesticides. They helped support sustainable crop protection. The shift away from synthetic chemicals encouraged new usage. In personal care, antimicrobial peptides targeted skin issues and hygiene needs. Beauty brands launched peptide-based formulations. Product innovation improved visibility in consumer markets. Although these segments held smaller shares, stable demand and research initiatives indicated long-term opportunities. They were expected to support future diversification of the market.
Route of Administration Analysis
In 2024, the Topical Section held a dominant market position in the Route of Administration Segment of Antimicrobial Peptides Market, and captured more than a 34.5% share. It was observed that demand increased because topical products act directly at the infection site. Localized treatment reduced systemic risks. Healthcare settings used these products widely for skin disorders. Post-surgical infection prevention supported usage. Hospitals relied on them in wound management. Rising cases of skin infections contributed to strong adoption. This route showed stable growth worldwide.
Industry analysts noted that the Oral Segment held a considerable share. Patient comfort supported uptake. Tablets and capsules provided simple treatment options. However, low bioavailability created challenges. The gastrointestinal environment reduced drug stability. Research efforts focused on new formulations. Improvements in drug protection technologies were expected to stimulate growth. Injectables also remained important. These products treated severe systemic infections. They delivered fast action. Physicians used them in hospital care. Rising antibiotic resistance supported continuous demand.
The Inhalation Segment demonstrated notable future potential. It was useful for respiratory infections. Non-invasive delivery improved adherence. Innovation in inhalable peptides progressed. Growth was linked to chronic lung diseases. Other routes such as nasal, ocular, and transdermal delivery served niche needs. These options helped when primary routes were unsuitable. Research continued to enhance effectiveness and penetration. Experts anticipated more product approvals. Overall, multiple administration routes supported market expansion. Resistance challenges increased the need for advanced antimicrobial peptide solutions.
End-User Analysis
In 2024, the Pharmaceuticals Section held a dominant market position in the End-User Segment of the Antimicrobial Peptides Market, and captured more than a 52.3% share. This position is linked to the strong use of peptide-based drugs. Growing resistance to antibiotics increased demand. Major companies invested in advanced formulations. Clinical trials expanded rapidly. Hospitals used these drugs for critical infections. The sector gained support from regulatory guidance. Its strong pipeline ensured product availability. As a result, pharmaceutical use remained the primary commercial driver of antimicrobial peptides worldwide.
The Biotechnology sector accounted for an important share. It is projected to grow steadily. Increased investment in genetic research supported demand. Biotech firms developed targeted therapies using peptides. Academic partnerships encouraged innovative experiments. The need for advanced antimicrobial platforms strengthened adoption. Enhanced research funding improved access to new solutions. Start-ups also entered the market. Their focus on resistant pathogens improved product development. The segment continued to gain recognition in therapeutic innovation and precision medicine applications.
Veterinary Clinics displayed moderate expansion. Animal treatment applications increased. Rising infection risks in livestock promoted product use. Better awareness of animal health raised adoption levels. Authorities supported safe peptide treatments. This improved trust among veterinarians. Other end-users include academic labs and diagnostic centers. These groups pushed research on antimicrobial resistance. Government grants helped new studies. Their contribution improved scientific progress. Continuous innovation from smaller users is expected to support the long-term growth of the antimicrobial peptides market.
Key Market Segments
By Type
- Cationic Peptides
- Linear Peptides
- Cyclic Peptides
- Alpha-Helical Peptides
- Beta-Sheet Peptides
- Other Peptides
By Source
- Natural Sources
- Synthetic Peptides
- Recombinant Peptides
- Engineered Peptides
By Application
- Healthcare & Therapeutics
- Agriculture & Animal Health
- Cosmetics & Personal Care
- Food & Beverages
- Veterinary Medicine
By Route of Administration
- Topical
- Oral
- Injectables
- Inhalation
- Others
By End-User
- Pharmaceuticals
- Biotechnology
- Veterinary Clinics
- Others
Drivers
Rising Antimicrobial Resistance Boosting AMP Adoption
Antimicrobial resistance is increasing worldwide. The effectiveness of traditional antibiotics is declining. This situation has created a need for new therapeutic solutions. Antimicrobial peptides are gaining attention due to their strong and broad antimicrobial abilities. They act against bacteria, viruses, and fungi. Their unique properties support faster action on resistant strains. The demand for AMPs is increasing as healthcare systems search for better treatments. This growth is driven by urgent global health challenges and limited treatment options.
AMPs use multiple mechanisms to kill pathogens. This lowers the chance of microbes developing resistance. Their structural diversity also helps them target a wide range of harmful organisms. These features make AMPs more effective than conventional antibiotics in many cases. Pharmaceutical companies are investing in AMP research. This investment supports the development of new drugs. The market potential is considered high. This is due to the growing burden of resistant infections in hospitals and communities.
The rise in AMR cases has become a major threat to public health. The World Health Organization has warned about a future without effective antibiotics. AMPs provide new hope in treating tough infections. They could replace or enhance current treatments. Their natural origin and safety profile are also driving adoption. Increased awareness and supportive policies are expected. These factors boost clinical development and commercialization. As a result, AMPs are becoming a vital solution in the fight against drug-resistant pathogens.
Restraints
Manufacturing, Safety, and Stability Constraints Limiting AMP Market Growth
The commercialisation of antimicrobial peptides (AMPs) is restricted due to major stability challenges. Natural AMPs are highly sensitive to proteolytic degradation. Their molecular structure is rapidly broken down inside the body. This reduces their therapeutic lifespan and limits clinical effectiveness. As a result, maintaining their stability during storage, formulation, and delivery becomes difficult. These limitations significantly weaken product performance. They also reduce the ability of pharmaceutical companies to scale AMPs into widely accepted therapeutic solutions.
Another critical restraint is toxicity concerns. AMPs may cause unwanted interactions with human cells. They can trigger immune reactions and create risks of cytotoxicity in patients. These safety issues demand extensive preclinical and clinical evaluations. Regulatory approval timelines are increased because more safety data is required. This situation slows pipeline progression. It creates uncertainty for investors and developers. The perception of potential adverse effects restricts broader clinical adoption and commercial confidence in AMP-based products.
High production cost also hinders AMP market growth. Their complex structures require expensive synthesis and purification techniques. Manufacturing at scale remains financially challenging. This results in higher product pricing and limited affordability. Cost inefficiencies discourage pharmaceutical manufacturers from large-scale investments. Competitive pressure from cheaper traditional antibiotics further slows market penetration. Until cost-effective production technologies advance, AMPs will struggle to achieve commercial competitiveness. This economic burden reduces accessibility and affects long-term market expansion opportunities.
Opportunities
Expanding Market Potential Through Advanced AMP Delivery and Biomaterial Integration
The expansion of engineered delivery systems is creating a strong opportunity for antimicrobial peptides (AMPs) in the healthcare market. Their integration into advanced carriers such as hydrogels, nanoparticles, and implant coatings increases targeted delivery. It also improves stability and reduces degradation issues. These technological improvements enhance therapeutic effectiveness and broaden the usage of AMPs in clinical settings. As a result, AMPs are becoming more competitive compared to traditional antibiotics in addressing resistant pathogens and complex infections. This advanced delivery focus is increasing industry investment and market adoption.
The development of multifunctional AMP-based biomaterials is expected to accelerate market growth. Embedding AMPs into medical device surfaces or wound care materials supports localized antimicrobial action. It further lowers infection risks associated with implants and surgical procedures. The growth of chronic wounds and hospital-acquired infections continues to increase demand for safer antimicrobial solutions. Regulatory encouragement for antibiotic alternatives supports this expansion. These innovations create additional value by combining infection prevention with improved healing outcomes.
Secondary markets, including medical devices, wound dressings, and preventive implant coatings, are projected to deliver new revenue streams for AMP developers. AMPs are positioned for strong uptake in specialized applications where conventional antibiotics have limitations. Companies working on scalable production and formulation improvement are likely to gain a competitive edge. Product diversification beyond therapeutic drugs helps reduce commercialization risks. The market opportunity is strengthened by increasing healthcare focus on advanced infection control strategies. This wider application landscape encourages strategic partnerships between pharmaceutical firms, biomaterial manufacturers, and device companies.
Trends
Transition Toward Computationally Engineered Antimicrobial Peptides
The antimicrobial peptides (AMPs) market is experiencing a shift toward computationally guided engineering. The adoption of rational peptide design and machine learning-based screening has increased significantly. These tools help identify promising AMP candidates faster and with greater accuracy. The focus is on designing peptides that target pathogens without harming host cells. This shift supports enhanced therapeutic performance. It also helps address resistance challenges associated with traditional antibiotics. AMPs designed using computation offer optimized features for specific clinical applications.
Bioengineering is strengthening AMP innovation. Modified peptide structures such as cyclic peptides and incorporation of non-natural amino acids are increasingly explored. These approaches enhance stability and improve selectivity. They also reduce toxicity risks that limit clinical success. Improved pharmacokinetics offers advantages in systemic therapies. Engineering allows customization against emerging drug-resistant bacteria. This trend demonstrates an industry movement from natural peptide discovery toward precision-driven development. It positions AMPs as viable pharmaceutical candidates.
The integration of advanced synthesis and design technologies is accelerating commercialization potential. Engineered AMPs can be produced more consistently and at scalable quality. This supports regulatory success and industrial adoption. The trend aligns with growing demand for next-generation anti-infective drugs. It also reflects strategic investment from biotech companies and research institutions. Better design tools reduce development costs and failure rates. As a result, the AMP pipeline is expanding. The market is expected to benefit from therapies tailored for real-world performance and healthcare needs.
Regional Analysis
In 2024, North America held a dominant market position, capturing more than a 39.7% share and holds US$ 97.5 Million market value for the year. The region continues to face a high and well-measured burden of antimicrobial resistance. According to the U.S. Centers for Disease Control and Prevention, more than 2.8 million drug-resistant infections occur each year, causing over 35,000 deaths. When Clostridioides difficile is included, cases exceed 3 million and deaths rise to 48,000. Treatment of six major pathogens costs more than US$ 4.6 billion annually. This burden strengthens demand for new antimicrobial options such as peptides.
Strong surveillance and reporting systems support market growth. The CDC has issued updated burden estimates for 2021 and 2022. These updates maintain urgency and improve visibility for sponsors and health providers. Robust surveillance also supports clinical trial design and hospital adoption. For Example, reliable national data guide endpoint selection for antimicrobial peptides. The presence of structured information flows enables informed purchasing and faster integration of new therapies in hospitals across the United States.
Regulatory and funding incentives further reinforce the regional lead. The U.S. Food and Drug Administration uses the GAIN Act to provide the Qualified Infectious Disease Product pathway, which includes Fast Track and five additional years of exclusivity. Public funding resources such as BARDA and the NIH have supported development programs. Study by CARB-X shows continued backing for novel antimicrobials, including peptides. These tools reduce development risk, attract investors, and accelerate commercialization opportunities in the antimicrobial peptides market.
The wider Americas also contribute to market uptake. A multicountry assessment estimated 141,000 deaths in 2019 were directly attributable to resistance and 569,000 were associated with it. Canada supports the landscape with coordinated surveillance and stewardship programs. For Instance, the Public Health Agency of Canada noted declining antibiotic use since 2019 but still identified 18.4% inappropriate hospital prescribing. This continued need for targeted therapies indicates favorable adoption potential. Overall, sustained clinical need, supportive policy, and reliable reporting explain North America’s leadership in the antimicrobial peptides market.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The antimicrobial peptides market is shaped by strong R&D investments and an increasing focus on drug-resistant infections. Companies such as AstraZeneca and Novartis are expanding research into biologics and peptide-based therapies. Their strategic focus includes respiratory, chronic, and hospital-acquired infections. These companies invest in early-stage clinical programs to improve the performance and stability of antimicrobial peptides. The growing interest in alternative therapies to conventional antibiotics strengthens the role of global pharmaceutical manufacturers in this market.
Sustained innovation drives leadership for Merck & Co., Inc. and Pfizer Inc. Their positions are supported by advanced technologies for combating multidrug-resistant pathogens. These companies emphasize immune-based approaches and peptide engineering. The market growth is attributed to their strong clinical development capabilities and global commercial networks. They support international collaborations to reduce antimicrobial resistance. Their focus on effective and safe peptide-based anti-infective solutions contributes to long-term expansion in the antimicrobial peptides market.
The market benefits from strong investments by Johnson & Johnson and Bayer AG, which are active in peptide therapeutics. These organizations strengthen their competitive advantage by improving peptide drug delivery and manufacturing processes. Increased emphasis on therapeutics targeting resistant bacterial strains improves product value. Their strategies include technology licensing, research agreements, and development of new modes of action. Their activities contribute to raising treatment efficiency. Both players are expected to expand their antimicrobial peptides portfolio to capture emerging opportunities in healthcare markets.
Several other global companies increase competitive intensity. Sanofi, GlaxoSmithKline (GSK), Boehringer Ingelheim, AbbVie, Eli Lilly and Co., Bristol-Myers Squibb, Roche, and Amgen Inc. invest in anti-infective biologics. These companies focus on precision medicine and peptide innovation. Product development initiatives target unmet clinical needs. The market expansion is supported by diversified pipelines and improved regulatory environments. Wider adoption of peptide-based antimicrobials is expected. Their efforts strengthen the global market outlook and support growth in antimicrobial drug discovery.
Market Key Players
- AstraZeneca
- Novartis
- Merck & Co. Inc.
- Pfizer Inc.
- Johnson & Johnson
- Bayer AG
- Sanofi
- GlaxoSmithKline (GSK)
- Boehringer Ingelheim
- AbbVie
- Eli Lilly and Co.
- Bristol-Myers Squibb
- Roche
- Amgen Inc.
- Other key players
Recent Developments
- In October 2023: Merck announced that it would collaborate, via the AMR Action Fund, investing ~$100 million over 10 years to support development of novel antibiotics (including peptide-based and other new modalities) with the aim of bringing 2–4 new antibiotics to patients by 2030.
- In October 2024: Pfizer entered into a collaboration with PATH and Bay Area Global Health Alliance to combat antimicrobial resistance in Senegal and Tanzania using an outcomes-based financing mechanism. The announcement was made on 4 October 2024.
- In February 2024: J&J announced that its investigational targeted oral peptide JNJ‑2113 (also referenced as JNJ-77242113) demonstrated positive Phase 2b results in moderate-to-severe plaque psoriasis. The trial (“FRONTIER 1”) showed that JNJ-2113 achieved the primary endpoint (PASI 75 at Week 16) and all secondary endpoints including PASI 100 of 40.5 % and IGA 0 (clear skin) of 45.2%.
Report Scope
Report Features Description Market Value (2024) US$ 245.6 Million Forecast Revenue (2034) US$ 776.5 Million CAGR (2025-2034) 12.2% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type (Cationic Peptides, Linear Peptides, Cyclic Peptides, Alpha-Helical Peptides, Beta-Sheet Peptides, Other Peptides), By Source (Natural Sources, Synthetic Peptides, Recombinant Peptides, Engineered Peptides), By Application (Healthcare & Therapeutics, Agriculture & Animal Health, Cosmetics & Personal Care, Food & Beverages, Veterinary Medicine), By Route of Administration (Topical, Oral, Injectables, Inhalation, Others), By End-User (Pharmaceuticals, Biotechnology, Veterinary Clinics, Others) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape AstraZeneca, Novartis, Merck & Co. Inc., Pfizer Inc., Johnson & Johnson, Bayer AG, Sanofi, GlaxoSmithKline (GSK), Boehringer Ingelheim, AbbVie, Eli Lilly and Co., Bristol-Myers Squibb, Roche, Amgen Inc., Other key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Antimicrobial Peptides MarketPublished date: Oct 2025add_shopping_cartBuy Now get_appDownload Sample
Antimicrobial Peptides MarketPublished date: Oct 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- AstraZeneca
- Novartis
- Merck & Co. Inc.
- Pfizer Inc.
- Johnson & Johnson
- Bayer AG
- Sanofi
- GlaxoSmithKline (GSK)
- Boehringer Ingelheim
- AbbVie
- Eli Lilly and Co.
- Bristol-Myers Squibb
- Roche
- Amgen Inc.
- Other key players










