Global Dyspnea Treatment Market By Treatment-Therapy(Supplemental Oxygen Therapy, Relaxation Therapy) Drugs(Antianxiety Drugs, Antibiotics, Anticholinergic Agents, Corticosteroids , Others) By Route of Administration(Oral, Inhalation) By End Users( Hospitals, Home Care, Specialty Centres, Other End-Users) and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Feb 2024
- Report ID: 83727
- Number of Pages: 268
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
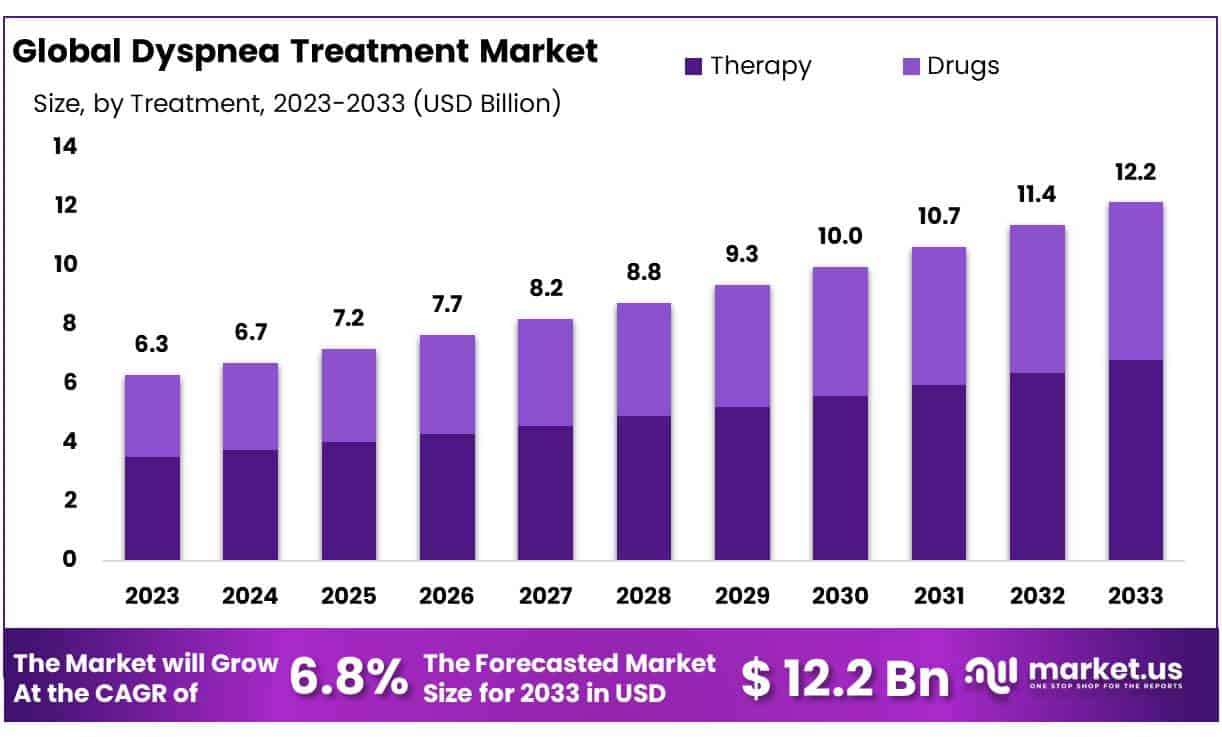
The Global Dyspnea Treatment Market size is expected to be worth around USD 12.2 Billion by 2033 from USD 6.3 Billion in 2023, growing at a CAGR of 6.8% during the forecast period from 2024 to 2033.
Dyspnea, defined as a feeling of breathlessness, is a common symptom of many different diseases and disorders. Dyspnea is a symptom that should not be ignored and can be treated with medication and other treatment options. It can be caused by respiratory problems, such as asthma or Chronic Obstructive Pulmonary Disease (COPD), or from certain heart conditions.
All of these factors result in the lungs not carrying enough oxygen to the body’s cells. Other causes could be something as simple as anemia due to lack of iron. It can be treated with medication such as bronchodilators and inhaled corticosteroids, along with other therapies such as oxygen, nebulizer treatments, and chest physiotherapy. Supplemental oxygen therapy should only be used when the patient has trouble breathing even though they are receiving sufficient oxygen. The patient’s physician should be informed before giving supplemental oxygen.
In the United States alone, as many as 25 million people suffer from dyspnea at some point in their lives. For the past 10 years, the global dyspnea treatment market has had a slow but steady rate of growth. On account of climate change and global warming, this market is expected to index rapid economic growth in years to come. To remain sustainable and viable, new technologies are being developed for those who are plagued by this disorder.
In February 2019, the Therapeutic Goods Administration (TGA) in Australia granted Mayne Pharma Group Limited approval for Kapanol (morphine sulphate pentahydrate), a low-dose sustained-release morphine capsule for the treatment of chronic dyspnea in palliative care patients with an advanced form of disease. The approval of Kapanol would help patients with severe COPD, heart failure, cancer, or other causes of chronic shortness of breath in palliative care.
Key Takeaways
- Market Size: Dyspnea Treatment Market size is expected to be worth around USD 12.2 Billion by 2033 from USD 6.3 Billion in 2023.
- Market Growth: The market growing at a CAGR of 6.8% during the forecast period from 2024 to 2033.
- Treatment Analysis: The therapies segment dominates 56% market share in 2023.
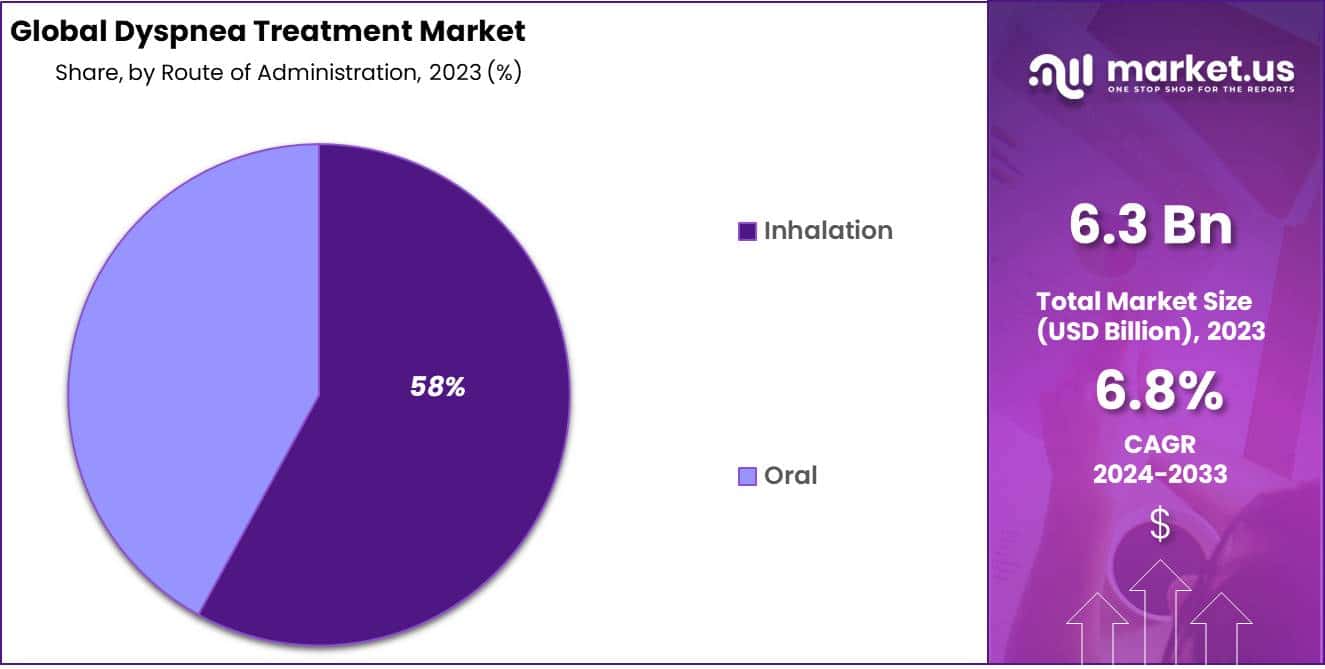
- Route of Administration Analysis: The inhalation remains the dominant route of administration with 58% market share.
- End-Use Analysis: The hospitals hold an impressive 39% market share for the dyspnea treatments market.
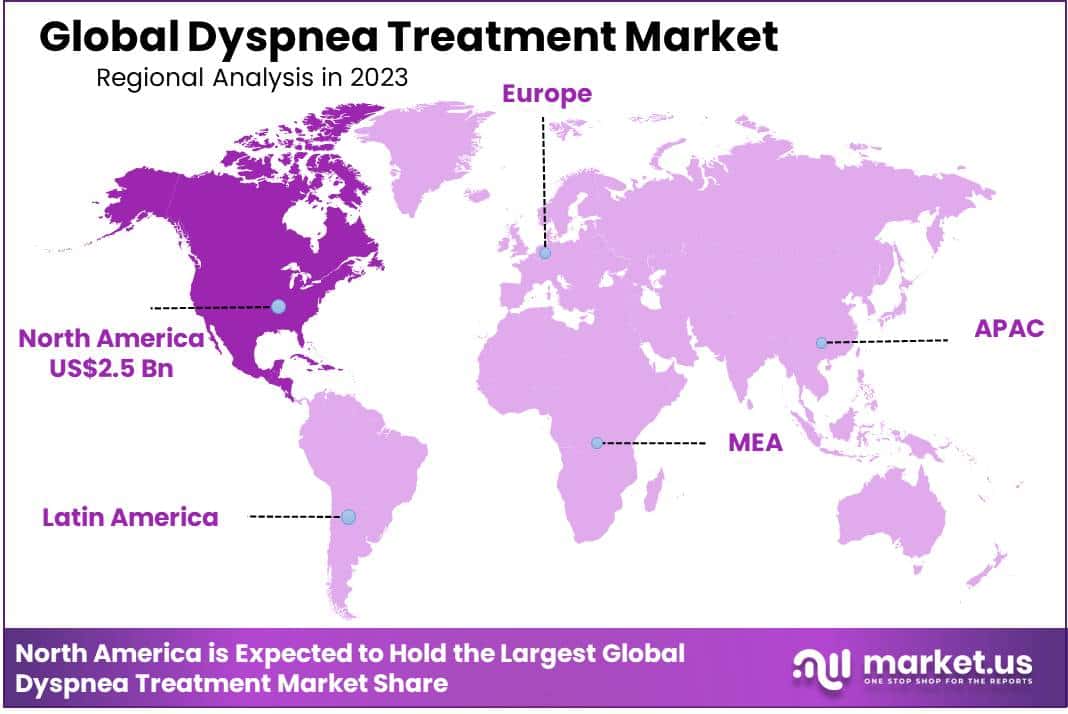
- Regional Analysis: North America held an estimated market share of 40% and held USD 2.5 billion market revenue in 2023.
Treatment Analysis
Dyspnea Treatment Market holds a substantial 56% market share among therapies, driven predominantly by pharmaceutical interventions. Dyspnea, or shortness of breath, presents an enormous global healthcare challenge and thus calls for effective therapeutic strategies. Pharmaceutical drugs play a critical role in managing dyspnea across its many causes – chronic obstructive pulmonary disease (COPD), asthma and interstitial lung diseases all cause dyspnea symptoms to manifest themselves; pharmaceutical interventions play an essential part in providing solutions.
At the forefront of this market segment are bronchodilators, corticosteroids, and combination therapies as they offer symptomatic relief, reduce airway inflammation and enhance lung function. Novel drug formulations and delivery systems continue to enhance treatment efficacy and patient compliance – key factors as respiratory diseases continue to affect populations as they age and exposure increases from environmental pollutants – fuelling steady market expansion in dyspnea treatment solutions; additionally ongoing research and development projects could bring forth innovative pharmacological solutions which offer cautious optimism for dyspnea management in future years.
Route of Administration Analysis
Dyspnea Treatment Market Inhalation remains the dominant route of administration with 58% market share; orally administered medications trail behind. Inhalation therapy plays an integral part in managing dyspnea by directly targeting lung airways to deliver medication like bronchodilators and corticosteroids to affected airways for rapid action with localized drug delivery resulting in decreased systemic side effects. This route offers several other advantages too such as rapid action onset timeframe and decreased systemic side effects.
Metered-dose inhalers (MDIs), dry powder inhalers (DPIs) and nebulizers have become increasingly popular as innovative delivery devices like metered-dose inhalers (MDIs) or metered dose inhalers with spacers improve drug deposition while increasing patient convenience; oral medications also play a crucial role in dyspnea management, particularly as long-term maintenance therapy or when inhalation therapy may not be feasible.
End Users Analysis
Hospitals hold an impressive 39% market share for dyspnea treatments, reflecting their central role in managing acute episodes and providing tailored care to individuals experiencing dyspnea. Hospitals serve as key centers for diagnosing and treating contributing conditions affecting dyspnea such as respiratory infections, heart failure and pulmonary embolism; furthermore they provide access to advanced imaging modalities as well as respiratory support devices like oxygen therapy or mechanical ventilation for treatment purposes.
Home care and specialty centers have emerged as essential elements in dyspnea management, catering to those needing long-term rehabilitation support and services. Home care allows individuals to receive tailored treatments right in their homes while improving independence and overall quality of life; specialty centers on the other hand provide multidisciplinary support tailored to dyspnea patients’ specific needs and offer comprehensive treatment plans designed around each unique dyspnea patient’s requirements. As healthcare delivery models continue to advance, collaboration among hospitals, home care providers, and specialty centers will play an essential part in optimizing dyspnea treatment outcomes and providing holistic patient care outcomes and holistic patient care outcomes for dyspnea patients alike.
Market Segments
Treatment
Therapy
- Supplemental Oxygen Therapy
- Relaxation Therapy
Drugs
- Antianxiety Drugs
- Antibiotics
- Anticholinergic Agents
- Corticosteroids
- Others
Route of Administration
- Oral
- Inhalation
End Users
- Hospitals
- Home Care
- Specialty Centres
- Other End-Users
Driver
Rising Prevalence of Chronic Respiratory Diseases
The increasing incidence of chronic respiratory conditions like chronic obstructive pulmonary disease (COPD), asthma and interstitial lung diseases is one of the major drivers for dyspnea treatment market growth. As people live longer lives due to lifestyle changes such as smoking or air pollution exposure and exposure to secondhand smoke contribute to respiratory health issues; leading to increased demand for effective dyspnea treatments which manage symptoms effectively in order to increase quality of life for affected individuals.
Technological Advancements in Respiratory Medicine
Technological innovations are fueling advancement in dyspnea treatment options. New drug formulations, inhalation devices, and diagnostic tools are constantly being created to address the specific needs of those experiencing dyspnea. Biologics and targeted therapies offer more precise approaches for specific respiratory conditions while advances such as telemedicine and remote patient monitoring enable healthcare providers to deliver personalized care and interventions directly to these individuals – furthering market expansion.
Trend
Shift towards Minimally Invasive Interventions
There has been an emerging shift towards minimally invasive interventions as an approach for managing dyspnea, driven by patient preferences for less invasive procedures with shorter recovery times. Procedures such as bronchoscopic lung volume reduction and endobronchial valve placement have become popular alternatives to open surgery for conditions like COPD and emphysema; offering relief without significant risks or complications as opposed to open procedures. Increasing adoption by both patients and healthcare providers alike
Integrating Digital Health Solutions
Integrating digital health solutions into dyspnea management has become a prominent trend, rapidly shaping the market landscape. Mobile applications, wearable devices and remote monitoring systems are being utilized to track symptoms and monitor lung function remotely while providing real-time feedback for both patients and healthcare providers. With expanding adoption of these digital tools it should simplify dyspnea management while increasing treatment adherence rates and ultimately leading to improved overall patient outcomes.
Restraint
High Cost of Advanced Therapies
The high costs associated with advanced dyspnea therapies present a major barrier to market growth. Innovative treatments such as biologics, gene therapies and targeted medications often carry high costs that are prohibitive to many in society, particularly in developing regions with limited healthcare resources. As expenses related to diagnostic tests, medical devices, and surgical procedures add further burdens on both individuals and healthcare systems, affordability issues limit widespread adoption of advanced dyspnea treatments – particularly among underserved populations – thus restricting market expansion.
Regulatory Challenges and Stringent Approval Processes
Stringent approval processes pose obstacles to the development and commercialization of dyspnea treatment options. As respiratory diseases are so complex, rigorous clinical trials and regulatory scrutiny must take place in order to ensure both safety and efficacy of new therapies. Regulator approval delays, compliance requirements and postmarket surveillance obligations can extend the time and costs to bring new treatments to market while raising development expenses for manufacturers. Furthermore, evolving regulatory environments and disparate reimbursement policies across different regions create uncertainty among industry stakeholders that impact investment decisions and market entry strategies.
Opportunity
Growing Emphasis on Personalized Medicine
With more and more doctors recognizing that personalized medicine offers significant opportunities, dyspnea treatment market holds great promise for growth. Advancements in genomic research, biomarker identification and precision medicine technologies have created opportunities for tailoring treatment approaches based on individual patient characteristics and disease phenotypes.
Utilising molecular profiling and predictive analytics, healthcare providers can efficiently divide patients suffering from dyspnea into subpopulations with unique therapeutic needs and response profiles. This allows healthcare providers to tailor interventions that optimize treatment outcomes while simultaneously mitigating side effects – driving demand for tailored dyspnea therapies and diagnostic solutions.
Expanding Healthcare Infrastructure in Emerging Markets
Emerging market healthcare infrastructure offers tremendous possibilities for market expansion in dyspnea treatment. Rapid urbanization, economic development and rising healthcare expenditures across Asia, Latin America and the Middle East is driving demand for improved respiratory care services and treatment solutions. Investments in healthcare facilities, medical technology and training programs are providing more people access to diagnose and treat respiratory conditions more quickly, creating a hospitable environment for market penetration by pharmaceutical companies, medical device makers and healthcare service providers.
By capitalizing on untapped markets in emerging economies industry players can exploit unrealized growth potential and diversify revenue streams in the global dyspnea treatment market.
Regional Analysis
North America held an estimated market share of 40% and held USD 2.5 billion market revenue in 2023, due to various factors, such as an aging demographic, rising smoking rates and environmental degradation. This region is experiencing an alarming surge in dyspnea cases and related respiratory and cardiac illnesses, prompting increased demand for effective therapies. Additionally, advancements in medical technology and pharmaceuticals are facilitating the creation of novel and more effective dyspnea treatment options. Growing awareness about dyspnea’s impact on quality of life combined with robust healthcare infrastructure and insurance coverage further drives North American dyspnea treatment market growth.
In each country-specific section of the dyspnea treatment market report, detailed insights are provided into individual market influencers and regulatory changes occurring domestically, which significantly shape both current dynamics and future trends. Key data points such as consumption volumes, production sites and capacities, import-export analyses, price trends, raw material costs and analyses of downstream and upstream value chains serve as critical indicators for forecasting the market outlook in each country.
Key Regions and Countries
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Russia
- Spain
- Rest of Europe
- APAC
- China
- Japan
- South Korea
- India
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- MEA
- GCC
- South Africa
- Israel
- Rest of MEA
Market Player Analysis
The competitive landscape of dyspnea treatment market offers comprehensive insights into each competitor. This includes providing an overview of each company, their financial status, revenue generation, market potential, research & development investments, new market strategies and global footprint. Furthermore, each data point relates directly to their activities and strategies within dyspnea treatment market.
Market Key Players
- Novartis AG
- Boehringer Ingelheim International GmbH
- Nephron Pharmaceuticals Corporation
- Cadila Healthcare Limited
- Mayne Pharma Group Limited
- Teva Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.
- Ligand Pharmaceuticals Incorporated
- Lupin Pharmaceuticals Inc.
- GlaxoSmithKline plc.
Recent Developments
- Novartis AG: Novartis has a strong presence in respiratory medicine with products like Xolair and Seebri Breezhaler, which could potentially be used for managing dyspnea related to certain conditions.
- Boehringer Ingelheim International GmbH: Partnered with Enanta Pharmaceuticals in October 2023 to develop and commercialize novel inhaled therapies for respiratory diseases, including potential applications for dyspnea.
- Nephron Pharmaceuticals Corporation: Primarily focuses on generic injectable and inhalation products, some of which could be used for managing symptoms associated with dyspnea.
- Cadila Healthcare Limited: Recently launched a generic version of Vilanterol/Fluticasone propionate inhaler, a medication used for COPD and asthma, which could help manage dyspnea in these patients.
Report Scope
Report Features Description Market Value (2023) USD 6.3 Billion Forecast Revenue (2033) USD 12.2 Billion CAGR (2024-2033) 6.8% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Treatment-Therapy(Supplemental Oxygen Therapy, Relaxation Therapy) Drugs(Antianxiety Drugs, Antibiotics, Anticholinergic Agents, Corticosteroids , Others) By Route of Administration(Oral, Inhalation) By End Users( Hospitals, Home Care, Specialty Centres, Other End-Users) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Novartis AG, Boehringer Ingelheim International GmbH, Nephron Pharmaceuticals Corporation, Cadila Healthcare Limited, Mayne Pharma Group Limited, Teva Pharmaceutical Industries Ltd., Bausch Health Companies Inc., Ligand Pharmaceuticals Incorporated, Lupin Pharmaceuticals Inc., GlaxoSmithKline plc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is dyspnea?Dyspnea is a medical term for shortness of breath or difficulty breathing, often characterized by a sensation of tightness in the chest or labored breathing.
How big is the Dyspnea Treatment Market?The global Dyspnea Treatment Market size was estimated at USD 6.3 Billion in 2023 and is expected to reach USD 12.2 Billion in 2033.
What is the Dyspnea Treatment Market growth?The global Dyspnea Treatment Market is expected to grow at a compound annual growth rate of 6.8%. From 2024 To 2033
Who are the key companies/players in the Dyspnea Treatment Market?Some of the key players in the Dyspnea Treatment Markets are Novartis AG, Boehringer Ingelheim International GmbH, Nephron Pharmaceuticals Corporation, Cadila Healthcare Limited, Mayne Pharma Group Limited, Teva Pharmaceutical Industries Ltd., Bausch Health Companies Inc., Ligand Pharmaceuticals Incorporated, Lupin Pharmaceuticals Inc., GlaxoSmithKline plc.
What are the common causes of dyspnea?Common causes of dyspnea include respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), pneumonia, and interstitial lung disease, as well as cardiac disorders like heart failure and arrhythmias.
How is dyspnea treated?Dyspnea treatment depends on the underlying cause and may include medications such as bronchodilators, corticosteroids, and diuretics, as well as supplemental oxygen therapy, pulmonary rehabilitation, lifestyle modifications, and surgical interventions in severe cases.
What factors contribute to the growth of the dyspnea treatment market?Factors driving the growth of the dyspnea treatment market include the increasing prevalence of respiratory and cardiac disorders, advancements in medical technology and pharmaceuticals, rising awareness about the impact of dyspnea on quality of life, and the availability of healthcare infrastructure and insurance coverage.

-
-
- Novartis AG
- Boehringer Ingelheim International GmbH
- Nephron Pharmaceuticals Corporation
- Cadila Healthcare Limited
- Mayne Pharma Group Limited
- Teva Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.
- Ligand Pharmaceuticals Incorporated
- Lupin Pharmaceuticals Inc.
- GlaxoSmithKline plc.










