Bleeding Control Tablets Market By Application (Hemophilia, Menorrhagia, Dysfunctional Uterine Bleeding, and Others), By End-user (Hospitals, Specialty Clinics, Retail Pharmacies, and Online Pharmacies), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Dec 2024
- Report ID: 134642
- Number of Pages: 325
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
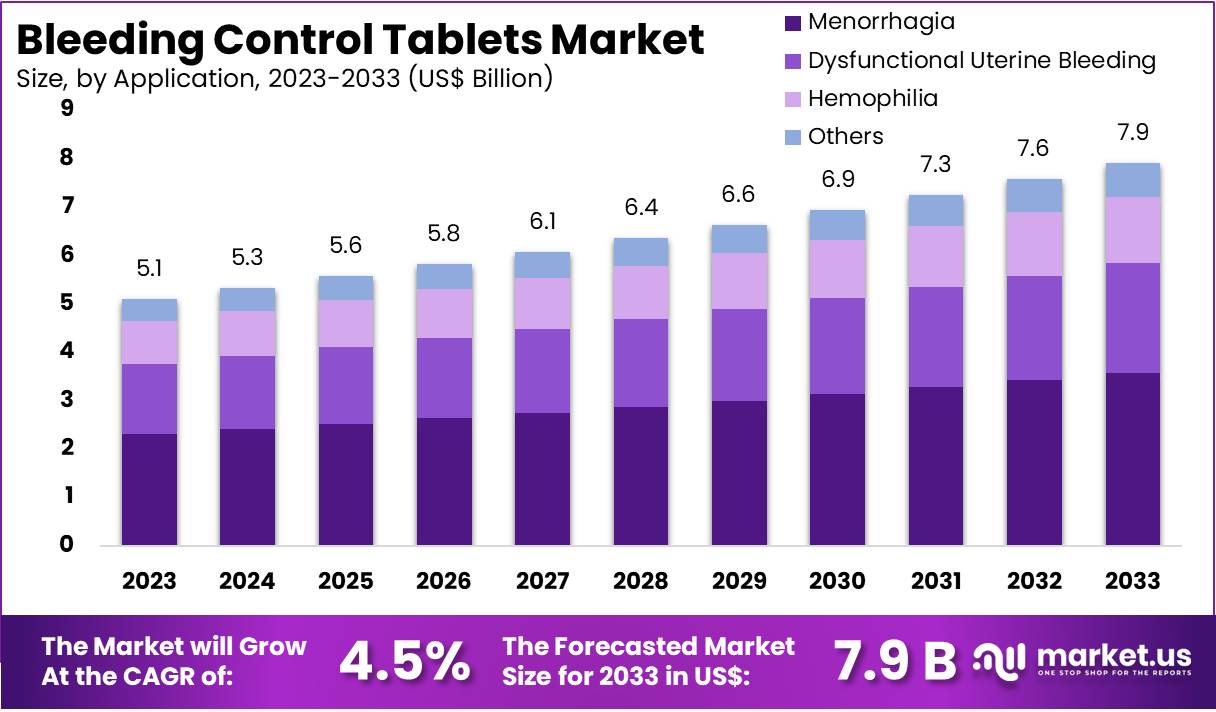
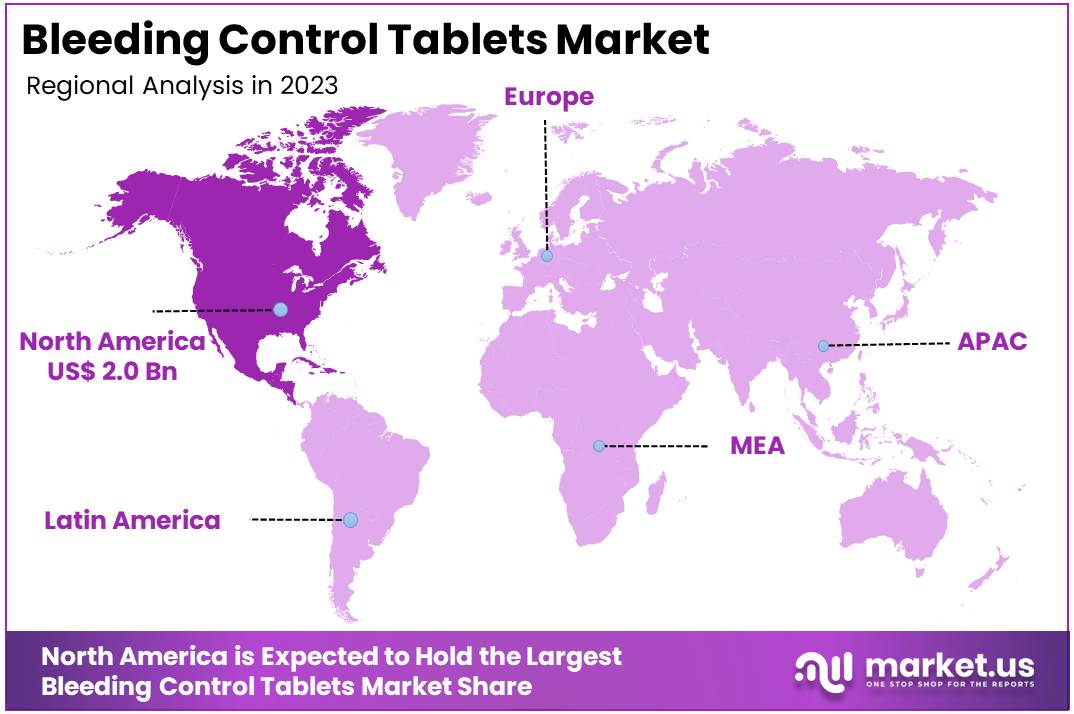
The Global Bleeding Control Tablets Market size is expected to be worth around US$ 7.9 Billion by 2033, from US$ 5.1 Billion in 2023, growing at a CAGR of 4.5% during the forecast period from 2024 to 2033. North America maintained a leading position in the market, securing over 39.6% of the market share, with a total market value of US$ 2.0 billion.
Rising concerns over emergency preparedness and trauma care are driving the growth of the bleeding control tablets market. These tablets, designed to quickly and effectively stop bleeding in trauma patients, are gaining traction in both emergency medical services and civilian applications. Bleeding control tablets are particularly valuable in situations where immediate access to professional medical care is not available, providing a critical tool for first responders, military personnel, and civilians.
In March 2024, the New Jersey Office of Homeland Security and Preparedness announced the distribution of bleeding control kits to houses of worship across the state, demonstrating a growing awareness of the need for public access to life-saving medical supplies. The kits, which include bleeding control tablets, aim to equip individuals to treat victims of potential active shooter incidents before first responders arrive. This initiative highlights a significant opportunity in the market, particularly in mass casualty events where rapid intervention can significantly reduce fatalities.
Additionally, increasing investments in first-aid training and public safety programs further drive demand for bleeding control products. Recent trends show a growing focus on portable, easy-to-use bleeding control solutions that can be easily stored in emergency kits, vehicles, and workplaces. Advancements in tablet formulations, designed to work rapidly in a variety of bleeding scenarios, also support market growth. With the expansion of trauma care awareness and a rising need for accessible emergency solutions, the bleeding control tablets market is poised to experience continued demand and innovation.
Key Takeaways
- In 2023, the market for bleeding control tablets generated a revenue of US$ 5.1 billion, with a CAGR of 4.5%, and is expected to reach US$ 7.9 billion by the year 2033.
- The application segment is divided into hemophilia, menorrhagia, dysfunctional uterine bleeding, and others, with menorrhagia taking the lead in 2023 with a market share of 45.2%.
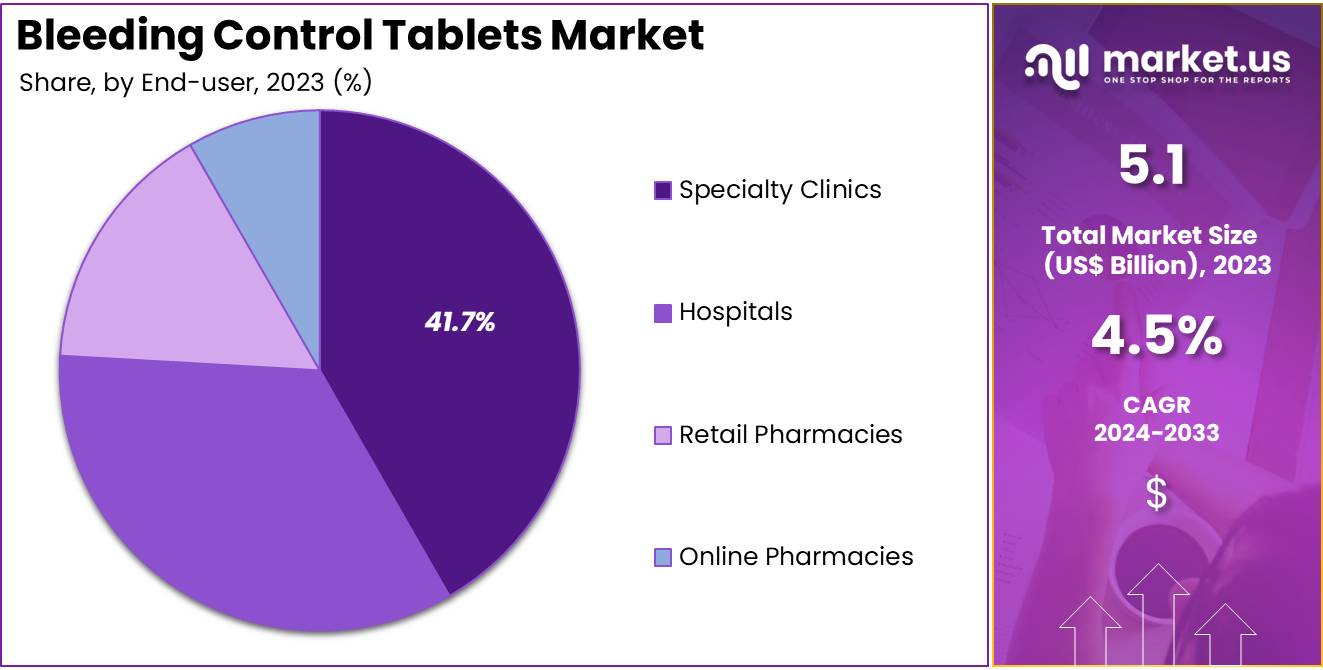
- Considering end-user, the market is divided into hospitals, specialty clinics, retail pharmacies, and online pharmacies. Among these, specialty clinics held a significant share of 41.7%.
- North America led the market by securing a market share of 39.6% in 2023.
Industrial Advantages For Market Key Players
Bleeding control tablets present significant business opportunities for key industry players, primarily through market expansion. These companies can access new and underserved markets, particularly in regions with high trauma incidence or limited medical facilities. This expansion enhances their market footprint, allowing them to reach a broader customer base and meet urgent healthcare needs.
Innovation in product offerings is another crucial benefit. Companies that develop or integrate bleeding control tablets into their portfolios can stay competitive by offering advanced emergency and trauma care solutions. This positions them as leaders in medical response innovations, a valuable trait in a competitive market.
Gaining regulatory approval for bleeding control tablets offers companies a significant competitive edge. It positions them as compliant and reliable, enhancing their brand reputation. Furthermore, strategic partnerships with healthcare providers, emergency response teams, and government bodies can solidify their market presence and expand their influence within the industry.
Lastly, bleeding control tablets contribute to cost-effectiveness in healthcare by reducing the expenses associated with managing bleeding emergencies. This cost reduction is appealing to healthcare facilities focused on budget efficiency. Additionally, by offering life-saving solutions, companies can attract a wider range of customers, including healthcare institutions and individual consumers for personal emergency kits.
Application Analysis
The menorrhagia segment led in 2023, claiming a market share of 45.2% owing to the rising prevalence of heavy menstrual bleeding among women of reproductive age. Menorrhagia, often caused by hormonal imbalances, fibroids, or other underlying conditions, is anticipated to drive demand for effective and non-invasive treatment options. The growing awareness of available treatments, including the use of bleeding control tablets, is likely to contribute to the segment’s expansion.
Furthermore, advancements in tablet formulations that provide targeted, faster-acting relief from heavy menstrual bleeding are projected to increase adoption among women seeking alternatives to surgical interventions. As more women seek to manage their symptoms and improve their quality of life, the menorrhagia segment is likely to witness continued growth within the broader bleeding control market.
End-user Analysis
The specialty clinics held a significant share of 41.7% as these clinics are increasingly becoming primary healthcare providers for conditions like bleeding disorders and heavy menstrual bleeding. The rise in outpatient care and the growing preference for non-invasive treatments are expected to drive demand for bleeding control tablets in specialty clinics. Patients seeking personalized, specialized care for conditions such as menorrhagia or other bleeding disorders are likely to choose specialty clinics for their treatments due to their focused expertise and convenience.
Additionally, the increasing number of specialty clinics that offer dedicated services for women’s health and hematology is expected to further fuel the adoption of bleeding control tablets. As more individuals seek specialized care outside of traditional hospital settings, specialty clinics are projected to play a key role in the growth of this market.
Key Market Segments
By Application
- Hemophilia
- Menorrhagia
- Dysfunctional Uterine Bleeding
- Others
By End-user
- Hospitals
- Specialty Clinics
- Retail Pharmacies
- Online Pharmacies
Drivers
Growing Prevalence of Sports-Related Injuries Driving the Market
The growing prevalence of sports-related injuries is significantly driving the demand for bleeding control tablets. A 2023 article from Johns Hopkins Medicine reported that approximately 3.5 million children aged 14 and under in the United States suffer from sports-related injuries each year. These injuries often result in cuts, abrasions, or other forms of trauma that lead to bleeding, creating a consistent need for effective bleeding control products.
The increasing participation in contact sports, as well as recreational activities among both children and adults, is expected to further escalate the number of injuries and, consequently, the need for rapid bleeding management solutions. Athletes, coaches, and emergency responders are likely to turn to these tablets to provide immediate treatment, especially in outdoor settings where access to medical care may be limited.
With an increasing number of injuries occurring both in professional sports and amateur settings, the market for bleeding control tablets is projected to expand significantly. The rising demand for portable, quick-to-use solutions for managing bleeding during sports events is expected to drive innovations in the development and use of bleeding control tablets.
Restraints
Stringent Regulatory Requirements Restraining the Market
The bleeding control tablets market faces significant restraints due to stringent regulatory requirements by authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These entities enforce rigorous testing and approval processes, which ensure product efficacy and safety but often delay market entry. Such requirements can be particularly challenging for smaller companies and startups, lacking the resources of larger pharmaceutical firms, thereby increasing the overall time and cost to market.
The approval process includes mandatory clinical trials, a step that further escalates costs and extends the timeline for bringing products to the market. These trials are crucial for demonstrating the safety and effectiveness of bleeding control tablets. However, they require substantial investment, making it difficult for smaller players in the industry to compete. The high costs associated with these trials can deter innovation and limit the diversity of products available to consumers.
Compliance with these regulatory standards is another hurdle, necessitating ongoing quality assurance and monitoring. This compliance burden can stifle innovation by consuming resources that could otherwise be used for product development and improvement. As a result, only well-established companies with ample resources can navigate these challenges effectively, potentially restricting market competition and innovation.
To mitigate these challenges, companies in the bleeding control tablets market need to engage proactively with regulatory bodies from the early stages of product development. By aligning their research and development efforts with regulatory expectations and investing in comprehensive pre-market testing, companies can streamline the approval process. Strategic engagement and compliance can thus play crucial roles in overcoming barriers to market entry and ensuring sustainable market growth.
Opportunities
Increasing Awareness About Postpartum Health Creating Opportunities
The increasing awareness about postpartum health, particularly regarding postpartum hemorrhage, presents a significant opportunity for the bleeding control tablets market. In March 2023, the World Health Organization (WHO) organized a summit on postpartum hemorrhage, highlighting the importance of available methods to control severe bleeding during childbirth.
As awareness of postpartum hemorrhage grows, there is an increasing demand for effective, easy-to-use solutions that can be used in both clinical and home settings to manage bleeding after childbirth. Bleeding control tablets can offer a vital solution for preventing and managing excessive postpartum bleeding, a condition that remains a leading cause of maternal morbidity and mortality worldwide.
The rising focus on maternal health, combined with ongoing efforts to improve the management of postpartum hemorrhage, is expected to lead to a greater adoption of these products. Furthermore, with healthcare systems around the world aiming to reduce maternal mortality, the introduction of new bleeding control treatments is likely to be met with a positive response, making this a promising area for market expansion.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors exert a notable influence on the bleeding control tablets market. Economic recessions often result in reduced healthcare budgets, limiting the accessibility and affordability of advanced medical solutions, including bleeding control tablets. In contrast, periods of economic growth typically lead to increased healthcare spending, which may boost the demand for life-saving products like these.
Geopolitical factors, such as trade restrictions, regulatory changes, or political instability, can disrupt global supply chains, affecting both the cost and availability of raw materials used in the production of bleeding control tablets. On a positive note, the growing frequency of trauma incidents, coupled with increasing awareness about the importance of rapid intervention, drives demand for bleeding control solutions.
Furthermore, advancements in tablet formulations and improved distribution networks continue to foster market growth. As the need for emergency care and first-aid products expands globally, the bleeding control tablets market is projected to continue its upward trajectory.
Trends
Increasing Launch of Training Programs Driving
The bleeding control tablets market is experiencing significant growth, driven by the increased emphasis on launching training programs. These initiatives aim to raise awareness about the importance of bleeding control and emergency medical intervention. A notable example from November 2023 is the Cintas Corporation’s collaboration with the American Heart Association (AHA). They introduced a Bleeding Control Training Program designed to educate businesses on handling severe blood loss effectively.
This training program covers essential topics such as scene safety, the use of personal protective equipment (PPE), and wound identification. It also provides practical hands-on training, including tourniquet application and wound packing techniques. Such comprehensive education is crucial in empowering individuals to manage life-threatening bleeding situations before professional help arrives.
As these training initiatives become more widespread, they are expected to further expand the market. By increasing public knowledge and encouraging the use of bleeding control tablets in various settings, these programs play a vital role in enhancing emergency preparedness. This not only boosts market growth but also contributes significantly to saving lives in critical situations.
Regional Analysis
North America is leading the Bleeding Control Tablets Market
North America dominated the market with the highest revenue share of 39.6% owing to a combination of increasing trauma incidents, rising awareness of emergency care, and the growing availability of advanced hemorrhage control products. Key to this growth was the May 2023 launch of the American College of Surgeons’ (ACS) “STOP THE BLEED” program, which focused on educating the public about essential bleeding control techniques.
This initiative has played a pivotal role in promoting the use of rapid-response products like bleeding control tablets, especially in emergencies where immediate intervention is critical. As the program gained traction, public demand for accessible, easy-to-use treatments increased, especially in situations where professional medical help may not be immediately available.
Additionally, the increasing focus on improving emergency response protocols across both urban and rural settings has driven up the adoption of bleeding control tablets, further contributing to market expansion. With more hospitals, emergency medical teams, and individuals becoming proactive in managing trauma-related bleeding, the market for these tablets has seen accelerated growth in the region.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is poised for rapid growth in the bleeding control market, driven by several key factors. Improvements in healthcare infrastructure and heightened awareness of trauma care are crucial elements. Additionally, the region is intensifying its focus on enhancing emergency medical services. These developments cater to the needs arising from large populations and frequent trauma-related incidents, such as traffic accidents and natural disasters.
In May 2022, Pfizer demonstrated its commitment to Asia by opening its first drug development laboratory at the Indian Institute of Technology in Chennai. This strategic expansion is aimed at fostering local innovation and enhancing the availability of advanced bleeding control treatments in the region. Pfizer’s presence is expected to have a significant positive impact on the pharmaceutical landscape across Asia.
The adoption of bleeding control solutions, such as tablets, is expected to surge in key Asian markets, including India, China, and Southeast Asia. As access to healthcare improves and emergency response systems become more robust, the demand for effective and user-friendly bleeding control options is likely to increase. This trend is anticipated to drive significant growth in the bleeding control market throughout Asia in the upcoming years.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The Asia Pacific region is projected to experience the fastest growth in the bleeding control market, driven by significant improvements in healthcare infrastructure. Increased awareness of trauma care and a concerted effort to enhance emergency medical services also contribute to this growth. The rising incidence of traffic accidents, natural disasters, and other trauma-related injuries in densely populated areas necessitates more efficient bleeding control solutions, fueling market expansion.
In May 2022, Pfizer further committed to this region by inaugurating its first drug development laboratory at the Indian Institute of Technology in Chennai. This establishment is a part of Pfizer’s strategy to deepen its roots in Asia’s pharmaceutical sector. The new facility is expected to boost local drug innovation and enhance the development and availability of bleeding control treatments.
As healthcare accessibility improves and emergency response systems are strengthened across key Asian markets, including India, China, and Southeast Asia, the demand for bleeding control tablets is anticipated to increase. This surge in demand is likely to drive significant market growth in the coming years, as these regions continue to invest in healthcare advancements and public safety measures.
Top Key Players in the Bleeding Control Tablets Market
- Tytek Medical
- Sanofi
- Pfizer
- Novartis
- Johnson & Johnson
- HemCon Medical Technologies
- Friedrich Bosch Medizintechnik
- CSL Behring
- Bayer
Recent Developments
- In January 2023: Tytek Medical, a manufacturer of medical supplies, provided a range of pre-hospital emergency trauma care products, including bandages, compressed gauze, chest decompression needles, shears, portable suction units, and others.
- In November 2022: Friedrich Bosch Medizintechnik announced its goal to expand its presence globally, aiming to be among the top three suppliers in its relevant markets by advancing every technology in every region.
Report Scope
Report Features Description Market Value (2023) US$ 5.1 billion Forecast Revenue (2033) US$ 7.9 billion CAGR (2024-2033) 4.5% Base Year for Estimation 2023 Historic Period 2019-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Application (Hemophilia, Menorrhagia, Dysfunctional Uterine Bleeding, and Others), By End-user (Hospitals, Specialty Clinics, Retail Pharmacies, and Online Pharmacies) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Tytek Medical, Sanofi, Pfizer, Novartis, Johnson & Johnson, HemCon Medical Technologies, Friedrich Bosch Medizintechnik, CSL Behring, and Bayer. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Bleeding Control Tablets MarketPublished date: Dec 2024add_shopping_cartBuy Now get_appDownload Sample
Bleeding Control Tablets MarketPublished date: Dec 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Tytek Medical
- Sanofi
- Pfizer
- Novartis
- Johnson & Johnson
- HemCon Medical Technologies
- Friedrich Bosch Medizintechnik
- CSL Behring
- Bayer










