Global Anesthesia Monitoring Devices Market By Product Type (Advanced Anesthesia Monitor, Basic Anesthesia Monitors, Integrated Anesthesia Monitors, Others), By End User (Hospitals, Ambulatory Surgical Centers), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: March 2024
- Report ID: 116520
- Number of Pages: 260
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
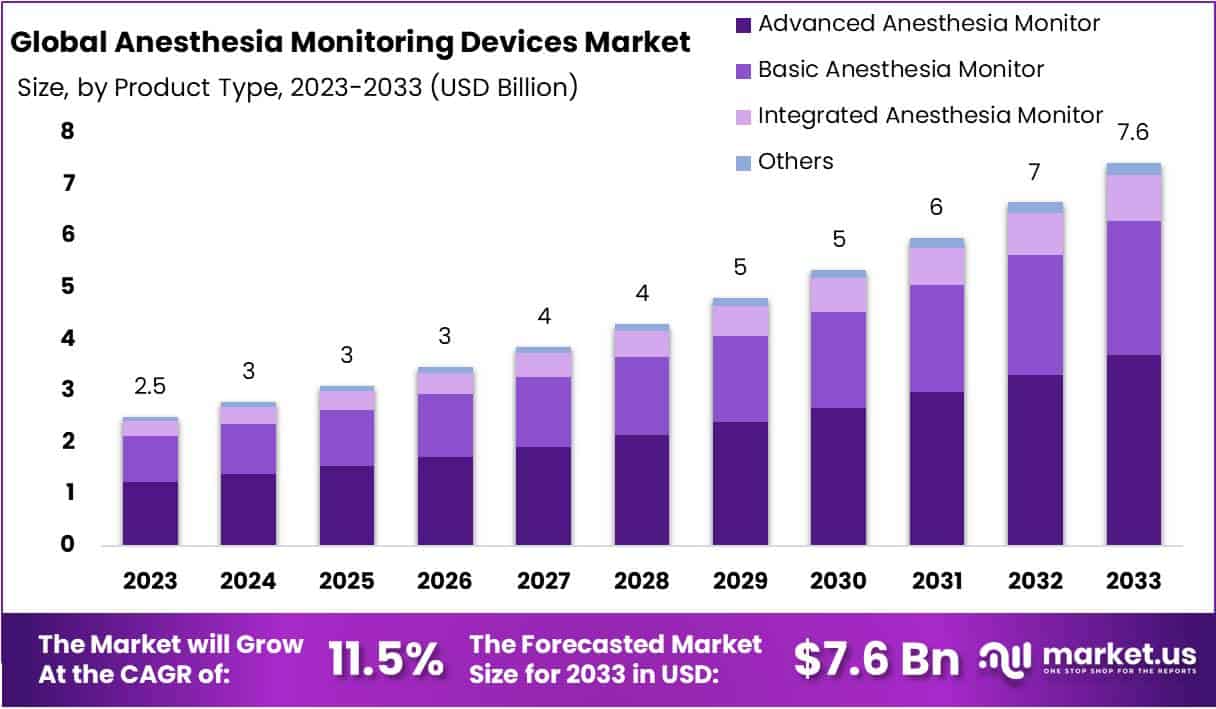
The Global Anesthesia Monitoring Devices Market size is expected to be worth around USD 7.6 Billion by 2033, from USD 2.5 Billion in 2023, growing at a CAGR of 11.5% during the forecast period from 2024 to 2033.
Anesthesia is a medication utilized to remove the sense of awareness or sensation while performing a medical procedure. It is broadly classified into 4 types, which is general anesthesia, regional anesthesia, sedation and local anesthesia. These are injected into the patients through injections, topical creams, sprays, inhalations and skin patches.
Anesthesia monitoring device encompass an inflatable blood pressure cuff usually strapped around upper arm and a pulse oximeter, attached to the finger, toe, or earlobe to measure oxygen level in blood. The usage of anesthesia monitoring devices usually relies on different types of cases performed in the operating room, such as assessment by measurement of patient’s oxygen, circulation, temperature and ventilation. Monitoring of patient has to be done keenly until the patient recovers from anesthesia. In addition, the dosage level of anesthetic drugs is monitored by anesthetist used on the patient.
Major factors boosting the market growth of anesthesia monitoring devices include rise in cases of surgeries across the globe coupled with escalating innovations and developments in technology for anesthesia delivery. In addition, rising elderly population more susceptible to diseases provides lucrative opportunities for the growth of anesthesia monitoring devices market.
Moreover, rising safety recognition amongst physicians combined with expediting volume of surgeries bolsters the position of market in recent year. However, the availability of conventional techniques for monitoring patients under anesthesia may cause impediment towards the growth of anesthesia monitoring devices market in the near future.
- For instance, each year a staggering 310 million major surgeries are performed, which is around 40 to 50 million in United States and 20 million in Europe.
Key Takeaways
- Based on product, advanced anesthesia monitors predominantly occupied a remarkable market share of 49.8% in the year 2023.
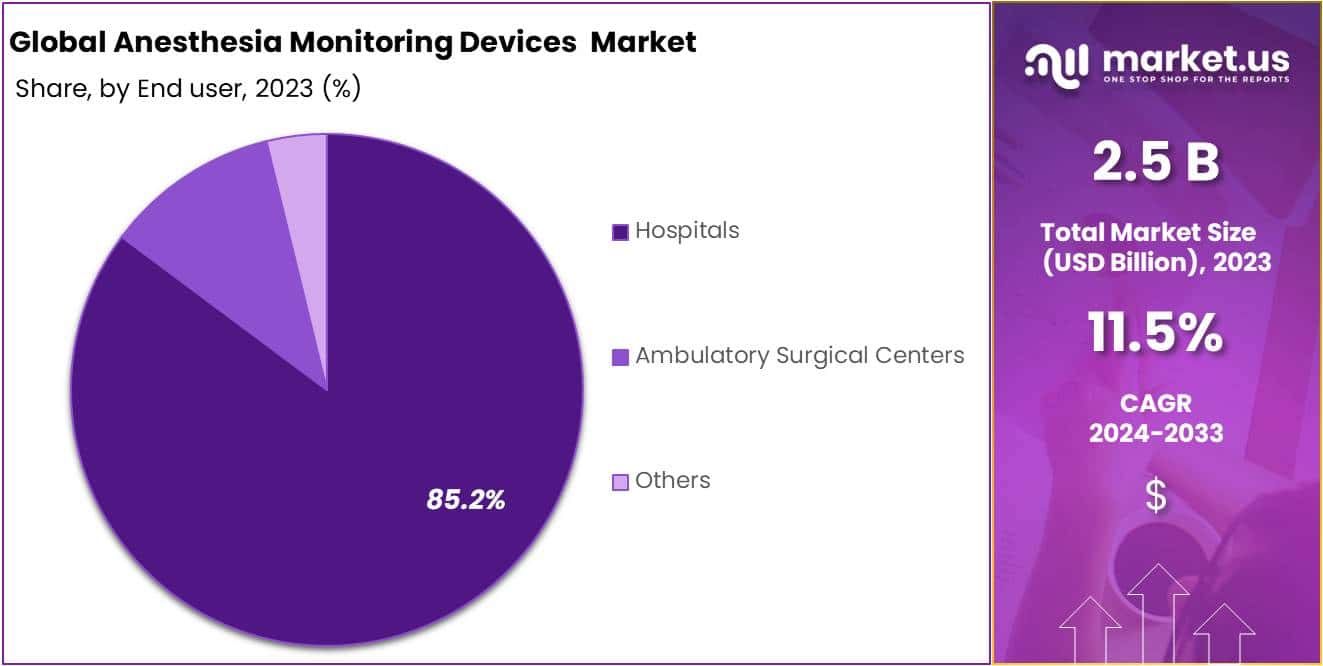
- Based on end use, hospital segment owing to the availability of highly advanced healthcare infrastructure and presence of skilled medical professionals captured an impressive market share of 85.2% in recent years.
- Rising pervasiveness of chronic illnesses such as diabetes, cancer and cardiovascular illnesses coupled with advancements in anesthesia monitoring devices, thrives the market to an unexpected pace.
- Anesthesia monitoring devices market is on an urge to hamper due to the diverse side effects associated with administering of anesthesia to patients.
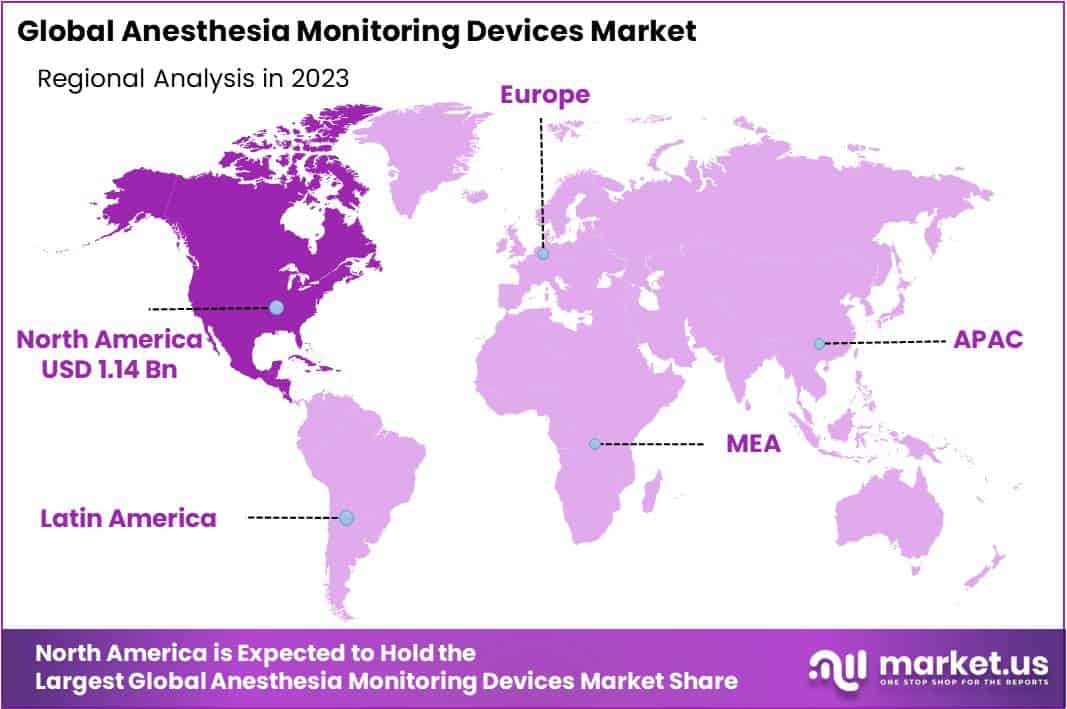
- North America leads the global Anesthesia Monitoring devices market in the year 2023, grabbing a large market share of 45.6%.
Product Type Analysis
Advanced anesthesia monitors dominate the market
Based on product, the market is broadly categorized into Advanced Anesthesia Monitor, Basic Anesthesia Monitors, Integrated Anesthesia Monitors and Other segments. A commendable market shares of 49.8% is captured by advanced anesthesia monitoring segment, dominating the market for anesthesia monitoring devices in the year 2023. Advanced anesthesia monitors are further segmented into standalone capnography monitors, gas monitors, and MRI compatible anesthesia monitors.
The segments’ growth is a reflection of the ongoing technological advancements allowing innovation in products, i.e., facilitating the development of highly trailblazing, durable and more precise advanced anesthesia monitors. The segment showcases further scale by virtue of integrating digitization, automation and technology in healthcare sectors.
In addition to the aforementioned factors, the market witnessed upward trajectory supported by hiking government expenditure leading to accelerated research and development of advanced medical technologies. Research and development expenditure by major market players is also anticipated to boost the market in the near future.
- MRI Compatible patient monitor MAGLIFE RT-1 was launched by Schiller in April 2022. The device allow close monitoring during scrutinization and can be completely controlled from outside the Faraday cage through MAGSCREEN RT-1.
End User Analysis
Notable market share is grabbed by the hospital segment
Based on end use of anesthesia monitoring devices, the market is bifurcated into hospitals, ambulatory surgical centers and other segments. An impressive market shares of 85.2% is withheld by hospital segment, dominating the market in the year 2023. The segment being predominant is highly ascribed to the availability of advanced infrastructure combined with existence of skilled healthcare professionals contributing towards the lucrative growth of the market.
On the other hand, a significant market portion is also captured by ambulatory surgical centers and further promises to excel during the prophecy period. Ambulatory surgical centers have heightened in numbers in recent times, falling into the category of Non-Operating room anesthesia, requiring well developed anesthesia systems. Many a times, these centers are prioritized over hospitals due to its provision of non-hospitalized outpatient experience saving patients’ time and money.
- According to MedPac analysis, number of ambulatory surgical centers escalated by 1.9% in 2014 in comparison to 2013.
Market Key Segments
By Product Type
- Advanced Anesthesia Monitor
- Basic Anesthesia Monitors
- Integrated Anesthesia Monitors
- Other
By End Use
- Hospitals
- Ambulatory Surgical Centers
- Others
Market Drivers
Rise in prevalence of chronic illnesses
The world is witnessing notable number of disease cases requiring hospitalization and surgery to carry out treatment, leading to increased number of surgeries across the globe. In addition, growing elderly population is the second major driving factor resulting into rising demand for anesthesia monitoring devices.
This is due to the fact of elderly population being more prone to chronic illnesses necessitating advanced surgical procedures involving anesthesia. Emerging economies are now focusing on safe healthcare practices creating ample opportunities for growth of anesthesia monitoring devices market.
- According to Pan American Health Organization update in 2023, approximately 20 million cancer cases were reported across the globe and cancer burden is likely to heighten by around 60% in the next upcoming two years. By 2040, cancer cases would increase up to 30 million in low and middle income countries.
Technological advancements in surgeries
The market for anesthesia monitoring devices is further thrived by advancing technologies, scientific breakthroughs and discoveries and coupled with product innovations leading to satisfactory patient surgical inventions, acting as a life saver for patients. An ultimatum for surgeries up scaled with the development of life-saving surgical procedures, minimal invasive surgeries and ameliorated patient outcomes. Thus, rising demand for anesthesia monitoring devices by virtue of increase surgical procedures robustly drives the market in recent years.
Market Restraints
Anesthesia associates side effects
Though these are ongoing benefits of anesthesia, but unfortunately it also associates some of the vital disadvantages for patients under surgeries. Patients being injected by anesthesia many a times undergo physical, mental and financial draining by virtue of the discomfort to the patients coupled with long hospital stays due to slow recovery. The side effects dealt by patients can be minor as well as major, where headaches, fever, nausea, body aches and fever forms minor side effects and major side effects may cause neurological damage to the patients.
Dearth of qualified anesthesiologists
Injecting or applying anesthesia to the patients is delicate science associating risks if not done appropriately. The doses have to be administered into the patient body depending upon how the body reacts in response to anesthesia. Thus, such crucial tasks require skilled professionals to avoid chances of failure, causing severe side effects. But limited number of qualified anesthesiologists in the healthcare sectors may hamper the market expansion.
Opportunities
Accelerating research and development
There is an ongoing research and development activities related to medical devices, systems, technologies, medications and equipment by virtue of growing demand for elective surgical procedures, aiming to ameliorate patient outcomes. There is an ongoing advancement in anesthesia monitoring devices for developing more advanced, sophisticated, precise, durable and reliable devices comprising additional features to make patient monitoring easy.
In addition, new startups have begun to deal with diverse types of anesthesia equipment, employing smart technologies to up scale the productivity coupled with efficacy in the firm. Thus, innovations of product combined with entry of new market players lead to the development of advanced anesthesia monitoring devices propelling the expansion of the market.
- Medasense Biometrics Ltd, a pain monitoring startup based in Israel announced that it has raised the USD 18 million in a Series C funding round in September 2020.
- In February 2022, Masimo for its product, Sedline’s advanced depth of anesthesia monitoring received FDA clearance to be used on patients below 1 year in the United States.
Latest Trends
The market is exclusively propelled by incorporating advanced technologies in anesthesia monitoring devices market. Some of these innovations include:
Implementation of Artificial Intelligence and Machine Learning
Anesthesia monitoring devices are now incorporated with artificial intelligence and machine learning, offering real time analysis of patient information, assisting anesthesiologists in taking informed decisions. Thus, the idea of these integrated technologies helps in forecasting adverse events, drug dosage optimization and anesthesia delivery personalization based on characteristics of patients.
Miniaturization and Portability
Ambulatory surgical centers and other remote locations are now integrated with Anesthesia monitoring devices by virtue of its compact and portable size. Thus portability of devices allows patients’ monitoring during transportation within healthcare settings.
Impact of Macroeconomic factors
Growing population is one of the most vital macroeconomic factor influencing the demand for anesthesia monitoring devices market. Primarily, rising population leads to an increased ultimatum for advanced surgical procedures. This is because, heightening cases of accidents, diseases and age related condition are recently reported.
Secondly, more resources are dispensed to healthcare services due to rise in healthcare spending, which directly results into higher investments in medical equipment such as anesthesia monitoring devices, thereby to make sure patient safety and quality care at the time of surgeries. Thus, rising population necessarily up scale the demand for anesthesia monitoring devices on a large scale.
Regional Analysis
North America Has Dominated the Anesthesia Monitoring Devices Market
Amongst all the geographies, North America came up as a frontrunner in the global anesthesia monitoring devices market occupying a noticeable market share of 45.6% in the year 2023. The dominance is accredited to the continual hike in the number of diseases such as cardiovascular problems, various types of cancers, and obesity cumulatively demanding surgeries.
- Cedars-Sinai data reports that coronary artery bypass graft surgery, is the most common heart surgery, with more than 300,000 successful bypass surgeries in United States.
- According to Center for Disease Control and Prevention, every 40 to 45 seconds, at least one individual suffers from heart attack, which is nearly 80,5000 of people dealing with heart attack each year.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Manufacturers in the market are actively implementing various strategic initiatives to establish a robust position and enhance their overall presence. These initiatives encompass partnership & collaboration, acquisition & mergers, and increasing investments in research and development (R&D). By expanding their R&D capabilities, key players can effectively develop and launch innovative products, thereby expanding their product portfolio and securing a strong market position.
Top Key Players in the Anesthesia Monitoring Devices Market
- BPL Medical Technologies
- Dragerwerk AG & Co.
- Heyer Medical AG
- Masimo
- Medasense Biometrics ltd
- Shenzhen Mindray Bio-Medical Electronics Co., ltd
- Neurowave Systems, Inc.
- Nonin
- Veterinary Anesthesia Systems, Inc.
- Allied Biotech Corporation
- FUKUDA DENSHI
- Schiller Americas
Recent Developments
- In March 2023: A product of Medasense Biometrics, ‘PMD-200 Monitor’, was approved by FDA. The device uses a wearable finger probe which monitors heart rate, sweat, blood pressure and movement of patients in order to determine their nociception level or physiological response to pain. This assists in analysis of data using machine learning, determining patients’ pain level.
- In November 2021: MDoloris Medical System for its HFVI MOC-9 high frequency variability index monitor was approved by Food and Drug Administration. The technology is the first ever monitoring solution for patients undergoing analgosedation and anesthesia.
Report Scope
Report Features Description Market Value (2023) USD 2.5 Billion Forecast Revenue (2033) USD 7.6 Billion CAGR (2024-2033) 11.5% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Advanced Anesthesia Monitor, Basic Anesthesia Monitors, Integrated Anesthesia Monitors, Others), By End User (Hospitals, Ambulatory Surgical Centers) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA. Competitive Landscape BPL Medical Technologies, Dragerwerk AG & Co., Heyer Medical AG, Masimo, Medasense Biometrics ltd, Shenzhen Mindray Bio-Medical Electronics Co., ltd, Neurowave Systems, Inc., Nonin, Veterinary Anesthesia Systems, Inc., Allied Biotech Corporation, FUKUDA DENSHI, Schiller Americas Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF).  Anesthesia Monitoring Devices MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample
Anesthesia Monitoring Devices MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- BPL Medical Technologies
- Dragerwerk AG & Co.
- Heyer Medical AG
- Masimo
- Medasense Biometrics ltd
- Shenzhen Mindray Bio-Medical Electronics Co., ltd
- Neurowave Systems, Inc.
- Nonin
- Veterinary Anesthesia Systems, Inc.
- Allied Biotech Corporation
- FUKUDA DENSHI
- Schiller Americas









