Quick Navigation
Overview
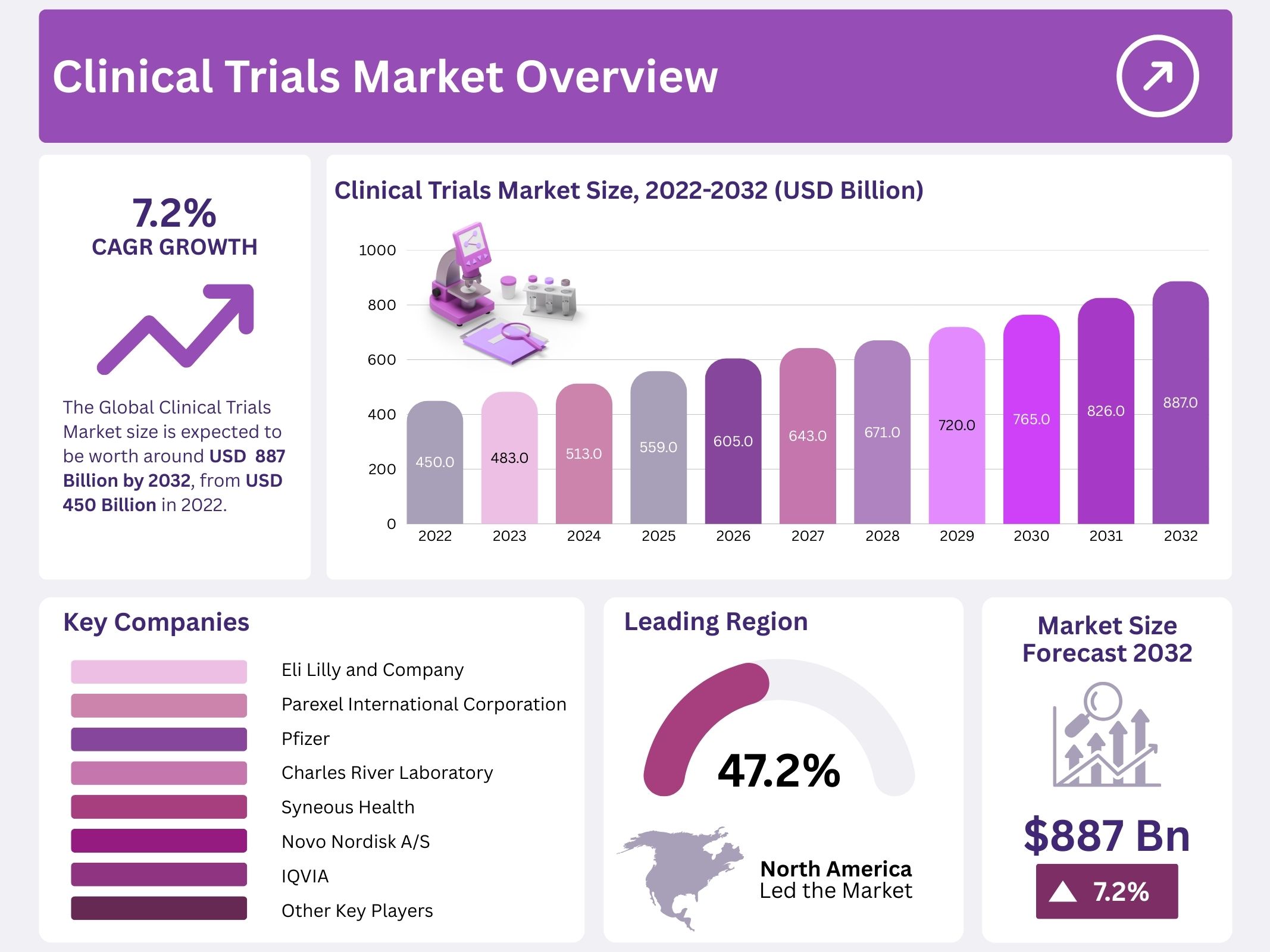
The global clinical trials market was valued at USD 450.1 billion in 2022 and is projected to reach USD 886.5 billion by 2032, expanding at a CAGR of 7.2% between 2023 and 2032. Clinical trials are research studies conducted on human subjects to evaluate the safety and effectiveness of new treatments, including drugs, devices, and dietary approaches. These trials play a critical role in advancing healthcare solutions and improving patient outcomes.
Market growth is supported by several key drivers. The globalization of drug development, rising prevalence of chronic diseases, and the increasing geriatric population have accelerated demand for clinical trials worldwide. Additionally, the use of online platforms to improve patient recruitment and participation has further supported market expansion. However, high operational costs and lengthy approval timelines remain significant challenges for the industry.
A notable trend is the rising outsourcing of clinical trial activities to contract research organizations (CROs). Pharmaceutical and biotechnology companies face high costs and low approval rates, making outsourcing a strategic approach to save time and resources. CROs offer specialized expertise and advanced technological solutions, enabling sponsors to focus on drug discovery and innovation. This shift is expected to strengthen the role of CROs in the global clinical trials landscape.
Government initiatives and global collaborations have also contributed to market development. For instance, the World Health Organization (WHO) launched the “Solidarity” trial to accelerate COVID-19 treatment research. Such international alliances highlight the importance of coordinated efforts in addressing global health crises and promoting advancements in clinical trial practices. These initiatives create opportunities for stronger global trial networks.
Despite existing challenges such as high costs and shortages of skilled personnel, the clinical trials market is expected to witness strong growth. Increasing adoption of digital tools, rising investment in clinical research, and supportive policies are likely to generate profitable opportunities. The ongoing demand for innovative therapies will continue to drive market expansion over the forecast period.
Key Takeaways
- The global clinical trials market is projected to expand from USD 450.1 billion in 2022 to USD 886.5 billion by 2032.
- The market is expected to grow at a compound annual growth rate (CAGR) of 7.2% during the forecast period of 2023–2032.
- Phase III trials generated the highest revenue share among all phases, indicating their pivotal role in overall clinical trial investments and expenditures.
- Phase II trials ranked second in revenue contribution, reflecting their critical importance in advancing promising therapies through mid-stage development.
- Oncology accounted for the largest revenue share among clinical trial indications, driven by rising cancer prevalence and strong demand for innovative therapeutic solutions.
- Pharmaceutical and biopharmaceutical companies dominated as end-users, holding the leading market position and are anticipated to sustain this dominance during the forecast period.
- North America captured 47.2% of the global clinical trials market in 2022, holding USD 212.4 billion in revenue.
- The region is projected to maintain its dominance throughout the forecast period, supported by advanced infrastructure, strong R&D investments, and supportive regulatory frameworks.
Regional Analysis
North America accounted for 47.2% of the global clinical trials market and is projected to retain its dominance during the forecast period. The adoption of advanced technologies in clinical trials and higher research and development spending are the main growth drivers in this region. Leading companies, such as PRA Health Sciences and IQVIA, are boosting the market expansion by integrating virtual services into different trial stages. Strong industry presence and continuous innovation will help North America maintain its leading market share.
Government initiatives also strengthen market growth in the United States. The U.S. Food and Drug Administration launched the Coronavirus Treatment Acceleration Program (CTAP) in March 2020. This program was designed to speed up the development of therapies targeting COVID-19 and related diseases. CTAP used all possible methods to deliver new treatments to patients quickly while ensuring their safety and efficacy. Supportive government policies, coupled with well-established regulatory frameworks, are expected to sustain the region’s market advantage in the coming years.
The Asia-Pacific region is forecasted to grow at the fastest CAGR of 6.8% during the assessment period. The availability of a large patient pool enables rapid recruitment for clinical trials, making the region highly attractive. Novotech, a leading biotech contract research organization in Asia-Pacific, has reported a surge in demand from biotechnology sponsors. COVID-19 trials in particular have driven research growth in the region. Many biotech firms prefer Asia-Pacific due to expedited processes and reliable trial outcomes, creating strong opportunities for future market expansion.
Segmentation Analysis
The Phase III segment accounted for the largest share of market revenue, owing to its high subject enrollment and longer treatment duration. This phase is recognized as the most expensive, requiring extended timelines and larger patient groups. Phase II followed as the second-largest revenue contributor. Its role is crucial, particularly in oncology research, where efficacy and dose optimization studies are conducted. Moreover, around 33% of drugs undergo Phase II trials, strengthening its market position. Several COVID-19 therapeutics in Phase II development further support growth.
The oncology segment dominated the global market in terms of revenue contribution and is expected to expand at the fastest CAGR. Rising pharmaceutical investment in preclinical and clinical oncology therapies, supported by the FDA and industry initiatives, drives this growth. Cardiovascular conditions are also projected to record strong CAGR during the forecast period. More than 190 drugs are currently in development, targeting conditions such as heart failure, stroke, and vascular diseases. Increasing demand for affordable treatments across low- and middle-income nations supports higher R&D spending.
Pharmaceutical and biopharmaceutical companies were the leading end-user segment and are expected to retain dominance. Rising cases of chronic and rare diseases, coupled with growing clinical trial activity, strengthen this leadership. Increased public and private sector investments in R&D also support market expansion. Clinical research organizations are anticipated to grow significantly, benefitting from rising outsourcing demand. However, the pandemic caused major disruptions in clinical trials worldwide, reducing patient enrollments by over 65% in 2020, with Japan, India, and the U.S. among the most affected regions.
Key Players Analysis
The global clinical trials market is witnessing rapid technological adoption, driving competitive intensity among service providers. To strengthen their offerings, major players are focusing on expanding capabilities and enhancing operational efficiency. For example, in January 2020, Wuxi AppTec introduced a fully integrated adeno-associated virus vector suspension platform to accelerate the development and commercialization of cell and gene therapies. Similarly, Acurian and Synexus launched SynexusPlus to streamline patient enrollment processes, reduce site burden, and increase trial efficiency, particularly significant during the pandemic period.
The industry is characterized by frequent mergers, acquisitions, and partnerships to sustain market presence and diversify service portfolios. Companies are leveraging advanced digital solutions to improve patient recruitment, site management, and regulatory compliance. SynexusPlus, a strategic solution introduced by Synexus under PPD, highlights the increasing focus on digital platforms for trial optimization. These efforts align with the broader trend of minimizing on-site operational challenges while maintaining trial quality and efficiency, reinforcing the sector’s adaptability to changing healthcare landscapes and regulatory requirements.
The market remains highly competitive, with several key players operating globally. Pharmaceutical Product Development, IQVIA, LLC, PAREXEL International Corporation, and Charles River Laboratory are among the major companies. Additional participants such as Eli Lilly and Company, Pfizer, Syneos Health, Novo Nordisk A/S, ICON Plc., and Wuxi AppTec are also prominent. The presence of these players, including Acurian and Synexus through SynexusPlus, ensures strong competition. Their strategic expansions, product innovations, and collaborations continue to shape the global clinical trials market landscape and contribute to accelerated healthcare advancements.
Conclusion
The clinical trials market is poised for steady growth, supported by rising demand for innovative therapies and expanding global collaborations. Growing cases of chronic diseases, an aging population, and increasing R&D investments are driving trial activities across multiple phases and therapeutic areas. Outsourcing to contract research organizations and the use of digital tools are transforming operations, improving efficiency, and reducing costs. While challenges such as high expenses and talent shortages remain, supportive government policies and international initiatives continue to strengthen the industry. Overall, clinical trials will remain a cornerstone of medical progress, providing opportunities for stakeholders and ensuring better patient outcomes worldwide.
Get in Touch with Us:
Market.us (Powered By Prudour Pvt. Ltd.)
Address: 420 Lexington Avenue, Suite 300, New York City, NY 10170, United States.
Contact No: +1 718 874 1545 (International), +91 78878 22626 (Asia).
Email: [email protected]
View More
Clinical Trial Imaging Market || Generative AI in Clinical Trials Market || Virtual Clinical Trials Market || Clinical Trial Supplies Market || Decentralized Clinical Trials (DCTS) Market || Clinical Trial Investigative Site Network Market || Pediatric Clinical Trials Market || Clinical Trial Biorepository and Archiving Solutions Market || In-Silico Clinical Trials Market || AI In Clinical Trials Market










