Global TCR-Based Antibody Market Analysis By Target Antigen (NY-ESO-1, p53, WT-1, Ebv), By Indication (Bladder Cancer, Multiple Myeloma, Ovarian Cancer, Nasopharyngeal Carcinoma, Other Indications), By End-Users (Hospitals, Specialized Clinics, Pharma & Biotech Research Laboratories, Gene Therapy Centers), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Feb 2024
- Report ID: 84335
- Number of Pages: 227
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
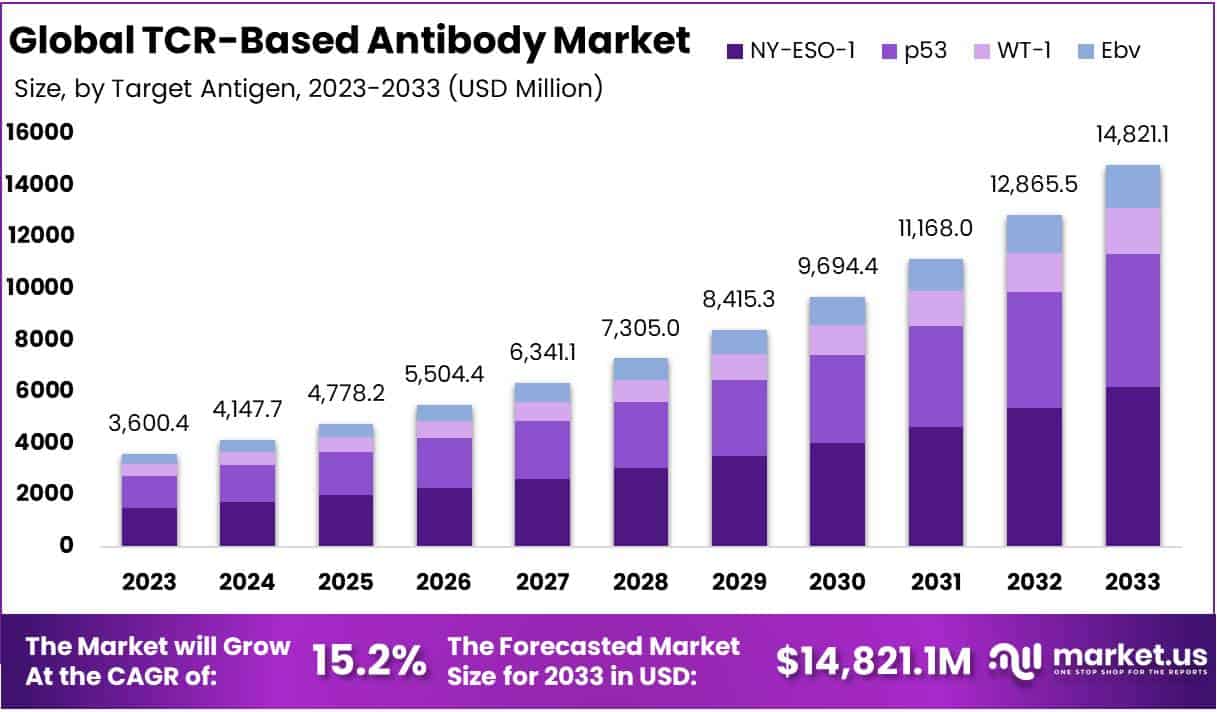
The Global TCR-Based Antibody Market size is expected to be worth around USD 14821.1 Million by 2033, from USD 3600.4 Million in 2023, growing at a CAGR of 15.2% during the forecast period from 2024 to 2033.
TCR-based antibodies are innovative therapeutic agents targeting cancer. These antibodies distinguish themselves by recognizing intracellular antigens presented by the MHC, unlike traditional antibodies that only target surface antigens. This capability broadens their application in cancer treatment, targeting antigens crucial for cancer cell survival.
The TCR-based antibody market is at a nascent stage but promises substantial growth due to the increasing demand for effective cancer therapies. Advances in biotechnology and significant investments in immunotherapy research drive this market. Furthermore, the rising global incidence of cancer necessitates novel treatment approaches, fueling interest in TCR-based therapies.
Regulatory bodies have expedited the development of these therapies through special designations, reflecting their potential. Collaborative efforts across pharmaceuticals, biotech, and academia are pivotal in overcoming challenges such as antigen identification and ensuring treatment safety. The TCR-based antibody market represents a promising frontier in cancer immunotherapy, aiming to deliver targeted and personalized treatment solutions.
Cancer immunotherapy, particularly TCR-based antibodies, represents a key advancement in oncology, especially in light of the American Cancer Society’s forecast of 1.8 million new cancer cases in the United States for 2023. The demand for innovative treatments has spurred companies to develop TCR-based therapies for various cancers, underscoring the sector’s growth and innovation potential. In the realm of autoimmune diseases, these therapies offer promising solutions for conditions like multiple sclerosis and rheumatoid arthritis by targeting specific disease-related antigens, opening avenues for targeted treatments.
The investments in 2023 showed strong interest in TCR-based therapies, with ImmuNext and ImmunityBio raising $165 million and $100 million, respectively, for their developments. This reflects a solid confidence in the transformative potential of TCR therapies across different diseases. Regulatory bodies like the FDA and EMA, along with the NCI’s support, highlight the government’s commitment to advancing this therapeutic approach.
Strategic industry movements, such as Gilead’s $22 billion acquisition of Immunomedics and partnerships like ImmunityBio with Bristol Myers Squibb, play crucial roles in accelerating TCR-based therapy development. The sector also faces challenges, including the complexity and cost of manufacturing high-quality therapies and the need for extensive trials to establish their safety and efficacy comprehensively.
Key Takeaways
- Market Growth: TCR-Based Antibody Market to reach USD 14,821.1 million by 2033, with a 15.2% CAGR from 2024-2033.
- Target Antigens: NY-ESO-1 dominates with 41.7% market share in 2023, followed by p53, WT-1, and EBV segments.
- Indications: Bladder Cancer leads with over 43% market share in 2023, followed by Multiple Myeloma and Ovarian Cancer.
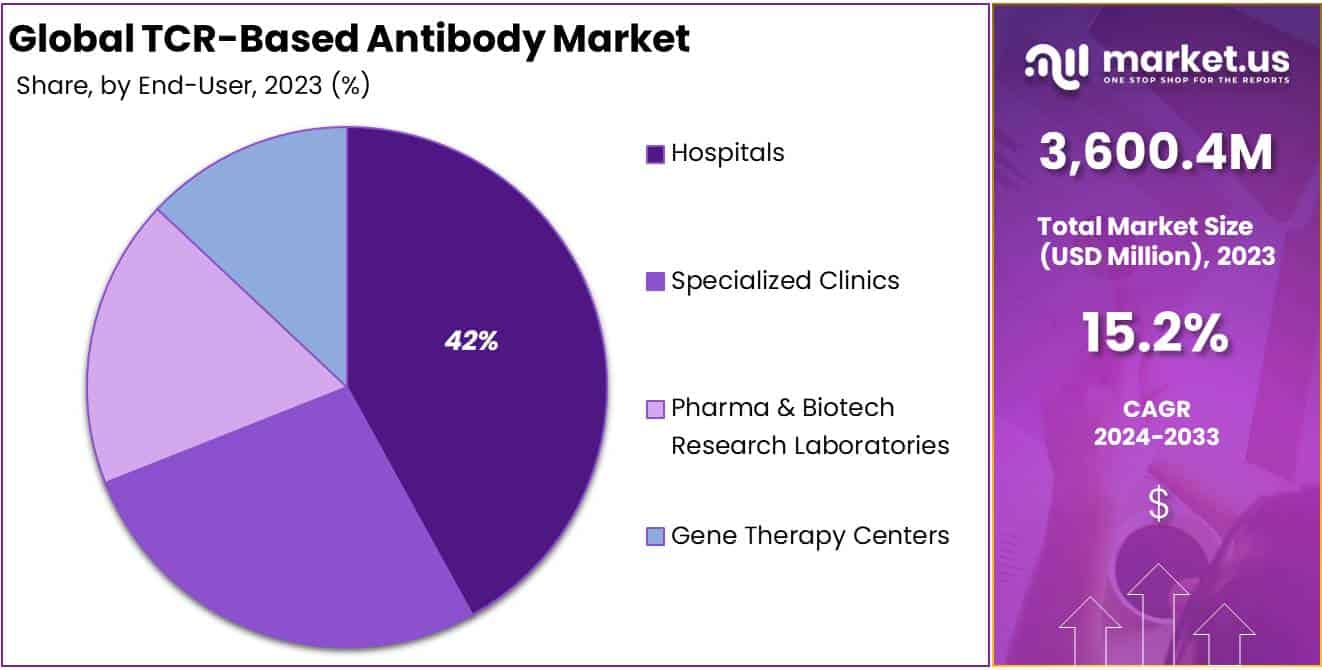
- End-Users: Hospitals hold 42% market share in 2023, followed by specialized clinics, pharma & biotech labs, and gene therapy centers.
- Drivers: Global cancer deaths spur demand, with over 10 million deaths annually and 50 million Americans living with autoimmune diseases.
- Restraints: High costs hinder market growth, with drug development costs exceeding $2.6 billion on average, limiting accessibility.
- Opportunities: Genomic sequencing advancements drive innovation, with cancer cases projected to rise by 70% over the next two decades.
- Trends: Collaborations surge by 30% in biopharma sector, expediting R&D and mitigating financial risks for innovative therapies.
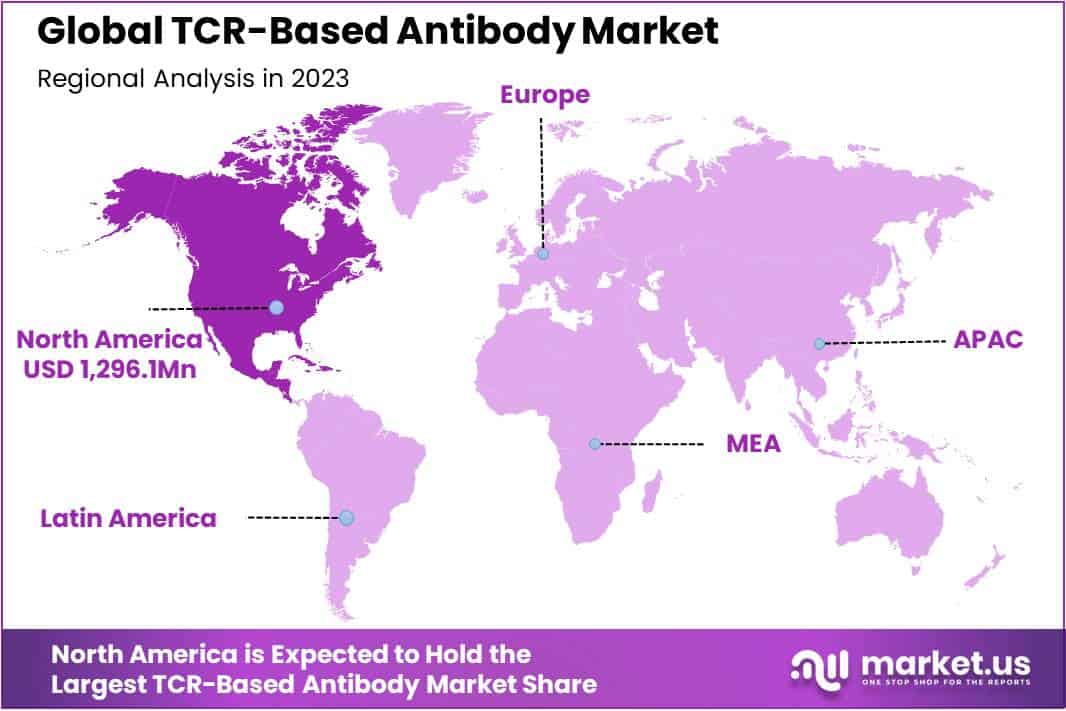
- Regional Dominance: North America leads with 36% market share in 2023, followed by Europe and Asia-Pacific.
Target Antigen Analysis
In 2023, the TCR-Based Antibody Market witnessed the NY-ESO-1 segment leading the target antigen category, commanding an impressive 41.7% market share. This segment’s success is largely due to its potential in cancer immunotherapy, targeting specific antigens present in various cancer types but not in normal tissues. Such precision in targeting makes NY-ESO-1 an ideal candidate for developing advanced TCR-based antibody therapies.
Following closely, the p53 segment has garnered significant attention within the market. Given its role in halting cancer progression and its mutation in about half of all human cancers, therapies targeting p53 present a groundbreaking approach. They aim to harness the immune system’s power to specifically target and destroy cancer cells with these mutations.
Another noteworthy segment is WT-1, associated with certain leukemias and solid tumors. The overexpression of WT-1 in these cancers offers a novel therapeutic target, promising more effective treatments for cancers with historically poor prognoses.
Lastly, the market is exploring therapies targeting Epstein-Barr Virus (EBV) antigens, which are linked to a variety of malignancies. This segment represents an innovative approach to treating EBV-associated cancers by utilizing TCR-based antibodies to target and eliminate cancer cells infected with the virus.
Indication Analysis
In 2023, the Bladder Cancer segment held a dominant market position in the Indication Segment of the TCR-Based Antibody Market, capturing more than a 43% share. This notable market share is primarily attributed to the increasing incidence of bladder cancer globally, coupled with the rising demand for targeted therapies. The effectiveness of TCR-based antibodies in recognizing and attacking cancer cells by targeting specific tumor antigens has driven significant interest and investment in this area, leading to a robust pipeline of therapeutic candidates and clinical trials specifically focusing on bladder cancer.
The Multiple Myeloma segment also showed substantial growth, driven by the unmet medical needs and the potential for TCR-based therapies to offer novel treatment options for patients. The segment’s growth is underpinned by ongoing research and development activities aimed at exploiting the unique nature of TCR-based mechanisms to target and eliminate myeloma cells more effectively than traditional treatments.
The Ovarian Cancer segment is another key area of focus within the TCR-Based Antibody Market. Despite being a smaller segment compared to Bladder Cancer and Multiple Myeloma, it is witnessing rapid growth due to the increasing prevalence of ovarian cancer and the critical need for more effective and personalized treatment options. The specificity of TCR-based antibodies in targeting tumor-specific antigens presents a promising approach to overcoming the challenges associated with treating ovarian cancer, leading to increased investment and clinical trials in this domain.
Nasopharyngeal Carcinoma and other indications collectively form an important part of the market, reflecting the broad applicability of TCR-based antibodies across a wide range of cancers. These segments are characterized by a diverse range of therapeutic candidates and research initiatives aimed at exploring the potential of TCR-based therapies in treating less common but highly aggressive forms of cancer.
End-Users Analysis
In 2023, the Hospitals segment held a dominant market position in the End-Users Segment of the TCR-Based Antibody Market, capturing more than a 42% share. This substantial market share is attributed to the critical role hospitals play in diagnosing and treating various cancers and autoimmune diseases, where TCR-based antibody therapies have shown significant potential. Hospitals, being at the forefront of patient care, have the infrastructure and specialized personnel required for administering such advanced treatments, which often involve complex protocols and monitoring.
Following closely, specialized clinics emerged as another significant segment, leveraging their expertise in targeted therapies to provide personalized treatment plans. These clinics offer a more focused approach to patient care, particularly for individuals with specific types of cancer or autoimmune diseases that can be addressed with TCR-based antibody therapies.
The Pharma & Biotech Research Laboratories segment also holds a pivotal position in the market, driving innovation in TCR-based antibody therapies. This segment’s growth is fueled by continuous research and development efforts aimed at discovering and refining new therapeutic antibodies. The collaborations between pharma and biotech companies with academic and research institutions further bolster this segment by speeding up the pace of innovation and clinical trials.
Gene Therapy Centers, although smaller in market share compared to hospitals and clinics, represent a rapidly growing segment. These centers specialize in delivering cutting-edge treatments, including TCR-based antibody therapies, for genetic disorders and certain types of cancer. Their role is increasingly crucial in the development and application of gene-editing technologies to enhance the efficacy and specificity of TCR-based therapies.
Key Market Segments
Target Antigen
- NY-ESO-1
- p53
- WT-1
- Ebv
Indication
- Bladder Cancer
- Multiple Myeloma
- Ovarian Cancer
- Nasopharyngeal Carcinoma
- Other Indications
End-Users
- Hospitals
- Specialized Clinics
- Pharma & Biotech Research Laboratories
- Gene Therapy Centers
Drivers
Rising Prevalence of Cancer and Autoimmune Diseases
The escalating incidence of cancer and autoimmune diseases globally serves as the principal catalyst propelling the growth of the Global TCR-Based Antibody Market. According to the World Health Organization, cancer accounts for nearly 10 million deaths annually, underscoring a dire need for innovative treatment modalities.
Concurrently, autoimmune diseases are on the rise, with the American Autoimmune Related Diseases Association reporting over 50 million Americans living with an autoimmune disease. This burgeoning patient population fuels the demand for advanced therapeutic solutions, particularly TCR-based therapies, renowned for their precision in targeting specific antigens present on malignancies or autoimmune-affected cells.
This targeted approach not only enhances treatment efficacy but also minimizes adverse effects, offering a tailored treatment paradigm. Consequently, the drive towards personalized medicine is intensifying research and development efforts in TCR-based therapies, positioning them as a pivotal frontier in addressing the growing healthcare challenge posed by cancer and autoimmune diseases.
Restraints
High Cost and Complexity of TCR-Based Therapy Development
The development and manufacturing of TCR (T-cell receptor)-Based therapies present significant financial and technical challenges, acting as a major restraint for the TCR-Based Antibody Market. The intricacies of these therapies necessitate advanced technological platforms and specialized manufacturing facilities, which entail substantial capital investments.
According to a report by the Biotechnology Innovation Organization, the average cost to bring a new drug from discovery to market exceeds $2.6 billion, a figure that underscores the financial hurdles in TCR-based therapy development. Furthermore, the complexity of engineering TCRs to precisely target specific cancer or autoimmune antigens increases the risk and cost of research and development.
This high cost structure can lead to expensive treatments, limiting patient accessibility and potentially curbing market expansion. As the industry grapples with these economic and technical obstacles, the affordability and scalability of TCR-based therapies remain critical challenges, impeding broader market growth and access.
Opportunities
Advancements in Genomic Sequencing and Bioinformatics
The opportunity presented by advancements in genomic sequencing and bioinformatics for the TCR-Based Antibody Market is underscored by compelling numerical data from leading healthcare organizations. The World Health Organization (WHO) has highlighted the increasing global burden of cancer, projecting new cancer cases to rise by about 70% over the next two decades.
This alarming statistic underscores the urgent need for innovative treatments like TCR-based therapies. Concurrently, the National Institutes of Health (NIH) has invested significantly in genomic research, with its budget for the National Human Genome Research Institute (NHGRI) reaching approximately $600 million in recent years. This investment supports the development of genomic sequencing technologies, which are crucial for identifying tumor-specific antigens with high precision.
Trends
Collaboration and Partnerships in Research and Development
In the Global TCR-Based Antibody Market, a prevailing trend is the burgeoning collaboration and partnerships across the spectrum of pharmaceutical companies, biotech entities, academia, and research institutions. This strategic convergence is designed to amalgamate resources, technological prowess, and specialized knowledge, thereby propelling the advancement of TCR-based therapeutic solutions.
A compelling illustration of this trend is evidenced by the 2021 report from the Biotechnology Innovation Organization, which highlighted a 30% increase in collaborative ventures within the biopharmaceutical sector, aimed specifically at enhancing oncology and immunotherapy research. These collaborative efforts not only expedite the R&D timeline but also distribute the financial and operational risks inherent in drug development, making the journey towards market readiness both efficient and cost-effective. By fostering such synergies, the industry is better positioned to navigate the complex landscape of TCR-based therapy development, ultimately accelerating the delivery of innovative treatments to patients worldwide.
Regional Analysis
In 2023, North America held a dominant market position, capturing more than a 36% share and holds USD 1,296.1 million market value for the year. This predominance is largely attributed to the region’s robust healthcare infrastructure, significant investments in biotechnology and pharmaceutical research, and a strong presence of leading market players engaged in TCR-based antibody development. The United States, as the central hub, spearheads innovation due to its comprehensive support mechanisms for biotech firms, including favorable regulatory frameworks and substantial financial backing for research and development (R&D) initiatives.
Europe emerged as the second-largest market, driven by its advanced healthcare systems, increased focus on personalized medicine, and supportive government policies promoting biopharmaceutical research. Countries such as Germany, the United Kingdom, and France are at the forefront, benefitting from substantial R&D investments, collaborative efforts between academic institutions and industry, and growing public-private partnerships. The market in Europe is further bolstered by the European Medicines Agency’s (EMA) proactive approach in encouraging innovative therapies, including TCR-based antibody treatments.
The Asia-Pacific region is identified as a rapidly growing market for TCR-based antibodies, with countries like China, Japan, and South Korea leading the charge. The growth is propelled by increasing healthcare expenditures, rising prevalence of cancer and other chronic diseases, and a growing emphasis on cutting-edge therapeutic modalities. Additionally, the region benefits from improving regulatory environments, which are becoming more conducive to the approval and commercialization of novel biologics. Strategic collaborations between Western biopharma companies and local entities are also facilitating technology transfer and localization of advanced therapies in the region.
Latin America and the Middle East & Africa (MEA) regions, while holding smaller shares of the global market, are anticipated to experience gradual growth. This is due to increasing awareness about advanced cancer therapies, expanding healthcare infrastructure, and rising investments in healthcare sectors. However, these regions face challenges such as limited access to sophisticated healthcare facilities, affordability issues, and a need for regulatory reforms to attract more investments in biopharmaceutical research.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the TCR-Based Antibody Market, several key players are driving innovation and progress. Lion TCR is known for its groundbreaking work in developing T-cell receptor therapies, focusing on personalized medicine and leveraging the body’s immune system against diseases.
GlaxoSmithKline (GSK) stands out with its vast resources and research capabilities, advancing TCR-based antibody treatments through a diverse pipeline and strategic partnerships. Adaptimmune Therapeutics PLC specializes in TCR-engineered T-cell therapies for cancer, emphasizing their commitment to targeting solid tumors. Celgene Corporation, now part of Bristol Myers Squibb, contributes its biopharmaceutical expertise to the market, particularly in hematologic malignancies and solid tumors.
Additionally, various other key players, ranging from established companies to startups, play pivotal roles in shaping the market’s dynamics through research, development, and commercialization efforts. Together, these players fuel advancements in precision medicine, offering hope for improved outcomes in oncology and beyond.
Market Key Players
- Lion TCR
- GlaxoSmithKline
- Adaptimmune Therapeutics PLC
- Celgene Corporation
- Immunocore
- Kuur Therapeutics Limited
- Lion TCR Pte. Ltd.
- Kite Pharma
- Takara Bio Inc.
- Ziopharm Oncology Inc.
- Merck & Co. Inc.
Recent Developments
- In January 2024, Merck & Co., Kite Pharma, and Celgene (now part of Bristol Myers Squibb), showcased their latest advancements in TCR-based therapies at the American Society of Hematology (ASH) Annual Meeting. These presentations highlighted ongoing Phase 1 and 2 clinical trials across various cancer types, indicating significant activity and progress within the market.
- In December 2023, Adaptimmune Therapeutics disclosed a collaboration worth $1.5 billion with Roche to advance TCR-T cell therapies for various cancer indications. The agreement encompasses co-development and profit-sharing arrangements for several pre-clinical and clinical-stage programs.
- In October 2023, Immunocore shared positive Phase 1 data for its lead TCR bispecific candidate, IMC-C103, at the European Society for Medical Oncology (ESMO) Congress. The data demonstrated promising safety and efficacy signals in patients with advanced solid tumors.
- In September 2023, Lion TCR and Takeda Pharmaceuticals announced a strategic collaboration aimed at developing and commercializing TCR-based therapies for solid tumors. The collaboration involved an upfront payment of $100 million and potential milestone payments exceeding $1 billion.
Report Scope
Report Features Description Market Value (2023) USD 3600.4 Mn Forecast Revenue (2033) USD 14821.1 Mn CAGR (2024-2033) 15.2% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Target Antigen (NY-ESO-1, p53, WT-1, Ebv), By Indication (Bladder Cancer, Multiple Myeloma, Ovarian Cancer, Nasopharyngeal Carcinoma, Other Indications), By End-Users (Hospitals, Specialized Clinics, Pharma & Biotech Research Laboratories, Gene Therapy Centers) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Lion TCR, GlaxoSmithKline, Adaptimmune Therapeutics PLC, Celgene Corporation, Immunocore, Kuur Therapeutics Limited, Lion TCR Pte. Ltd., Kite Pharma, Takara Bio Inc., Ziopharm Oncology Inc., Merck & Co. Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the size of the TCR-Based Antibody market in 2023?The TCR-Based Antibody market size is USD 3600.4 million in 2023.
What is the projected CAGR at which the TCR-Based Antibody market is expected to grow at?The TCR-Based Antibody market is expected to grow at a CAGR of 15.2% (2024-2033).
List the segments encompassed in this report on the TCR-Based Antibody market?Market.US has segmented the TCR-Based Antibody market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Target Antigen the market has been segmented into NY-ESO-1, p53, WT-1, Ebv. By Indication the market has been segmented into Bladder Cancer, Multiple Myeloma, Ovarian Cancer, Nasopharyngeal Carcinoma, Other Indications. By End-Users the market has been segmented into Hospitals, Specialized Clinics, Pharma & Biotech Research Laboratories, Gene Therapy Centers.
List the key industry players of the TCR-Based Antibody market?Lion TCR, GlaxoSmithKline, Adaptimmune Therapeutics PLC, Celgene Corporation, Immunocore, Kuur Therapeutics Limited, Lion TCR Pte. Ltd., Kite Pharma, Takara Bio Inc., Ziopharm Oncology Inc., Merck & Co. Inc.
Which region is more appealing for vendors employed in the TCR-Based Antibody market?North America is expected to account for the highest revenue share of 36% and boasting an impressive market value of USD 1296.1 million. Therefore, the TCR-Based Antibody industry in North America is expected to garner significant business opportunities over the forecast period.
Name the key areas of business for TCR-Based Antibody?The US, Canada, India, China, UK, Japan, & Germany are key areas of operation for the TCR-Based Antibody Market.

-
-
- Lion TCR
- GlaxoSmithKline
- Adaptimmune Therapeutics PLC
- Celgene Corporation
- Immunocore
- Kuur Therapeutics Limited
- Lion TCR Pte. Ltd.
- Kite Pharma
- Takara Bio Inc.
- Ziopharm Oncology Inc.
- Merck & Co. Inc.










