Systemic Inflammatory Response Syndrome Treatment Market By Indication (Urinary Tract Infection (UTI), Autoimmune Diseases, Pneumonia, and Others), By End-user (Hospitals, Ambulatory Surgical Centers, Specialty Clinics, and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: July 2025
- Report ID: 153472
- Number of Pages: 302
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
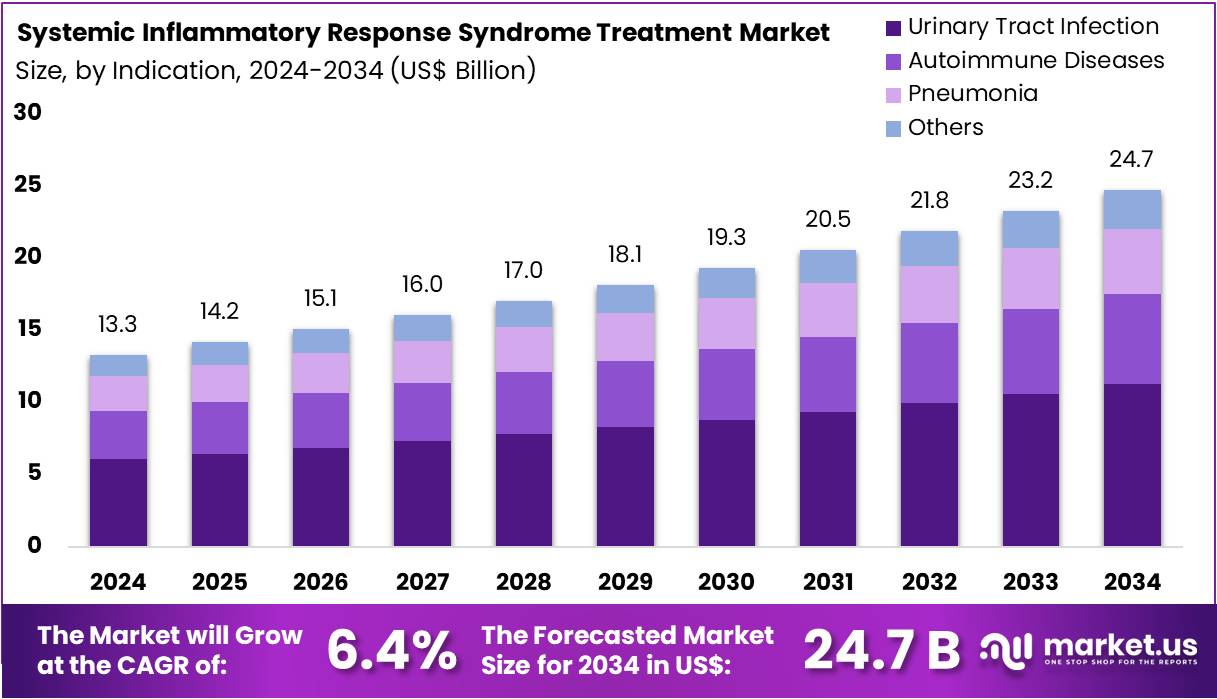
The Systemic Inflammatory Response Syndrome Treatment Market size is expected to be worth around US$ 24.7 billion by 2034 from US$ 13.3 billion in 2024, growing at a CAGR of 6.4% during the forecast period 2025 to 2034.
Increasing cases of systemic inflammatory response syndrome (SIRS) due to infections, trauma, and surgeries are driving the growth of the SIRS treatment market. SIRS is a severe, life-threatening condition characterized by widespread inflammation throughout the body, often leading to organ failure if not managed effectively. The rise in chronic diseases, such as diabetes, cardiovascular conditions, and respiratory infections, which increase the risk of SIRS, has further contributed to the growing need for effective treatments.
Current therapeutic approaches focus on addressing the underlying causes of inflammation, such as antibiotics for infections and immunomodulatory agents to control the body’s immune response. Additionally, advancements in biomarkers and early detection methods are creating new opportunities to improve the diagnosis and treatment of SIRS at its onset.
In October 2023, the Center for Cellular and Molecular Platforms (C-CAMP) in Bengaluru and the Institute of Life Sciences in Bhubaneshwar granted a license for their innovative sepsis treatment, SUR-101, to SurvivX, a biotech company based in the Netherlands. This partnership highlights a growing trend of research into novel biologics and therapies targeting inflammation pathways to more effectively manage SIRS and its complications.
The market is also seeing an increase in the development of combination therapies that can simultaneously address multiple factors contributing to SIRS, improving patient outcomes. As healthcare providers focus more on personalized medicine and early intervention, the SIRS treatment market is poised for significant advancements, presenting new opportunities for innovative treatments and improved management of this complex condition.
Key Takeaways
- In 2024, the market for systemic inflammatory response syndrome treatment generated a revenue of US$ 13.3 billion, with a CAGR of 6.4%, and is expected to reach US$ 24.7 billion by the year 2034.
- The indication segment is divided into urinary tract infection, autoimmune diseases, pneumonia, and others, with urinary tract infection taking the lead in 2023 with a market share of 45.6%.
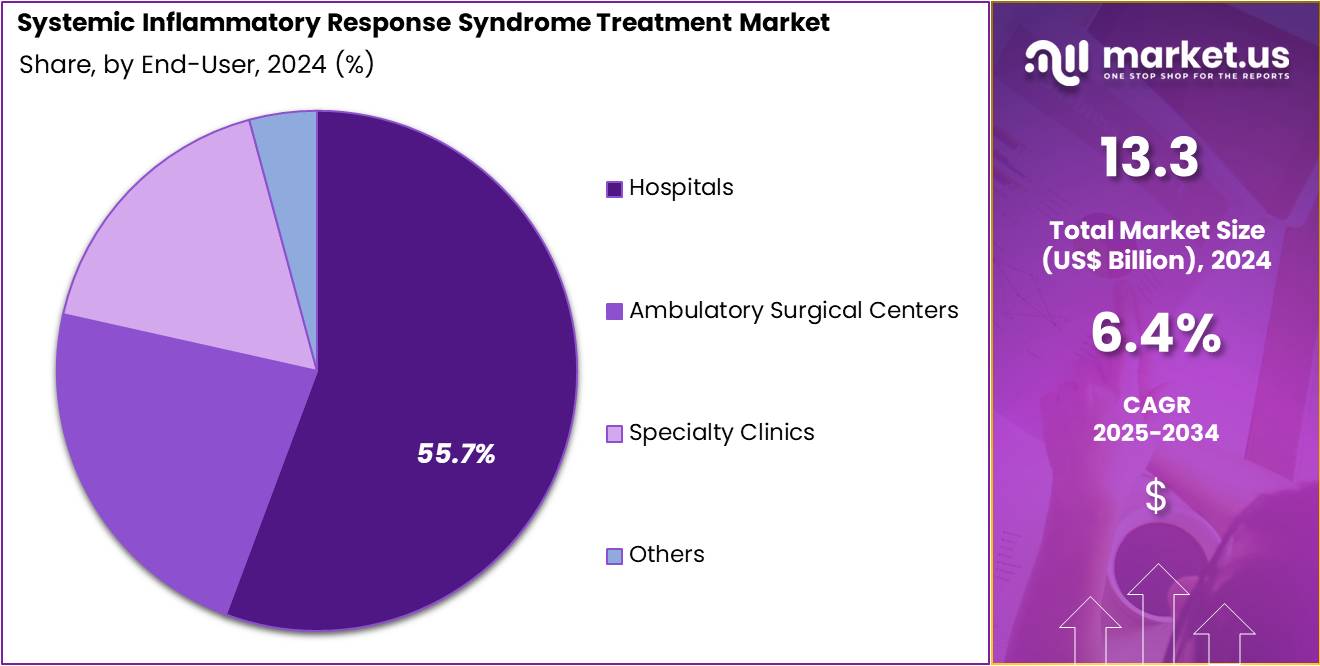
- Considering end-user, the market is divided into hospitals, ambulatory surgical centers, specialty clinics, and others. Among these, hospitals held a significant share of 55.7%.
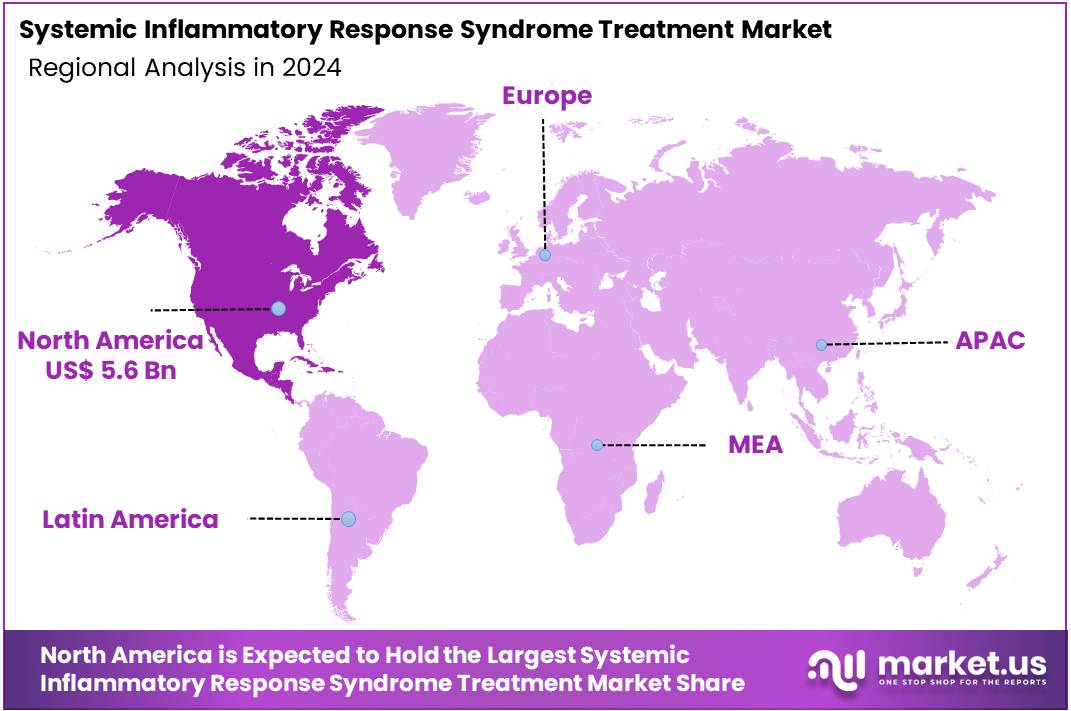
- North America led the market by securing a market share of 41.8% in 2023.
Indication Analysis
Urinary tract infection (UTI) holds the largest share of 45.6% in the indication segment of the systemic inflammatory response syndrome (SIRS) treatment market. This growth is expected to continue due to the high prevalence of UTIs worldwide, particularly among women, the elderly, and individuals with underlying health conditions such as diabetes.
UTIs are often associated with significant morbidity, leading to increased demand for effective treatments. The rising number of patients with urinary tract infections, coupled with the growing focus on early intervention and treatment, is projected to drive demand for SIRS treatments targeting UTIs. The increasing resistance of bacteria to antibiotics has also heightened the need for novel therapies that can manage the systemic inflammation triggered by severe infections like UTIs.
Furthermore, as healthcare providers focus more on preventing complications from UTIs, such as sepsis, the demand for timely and efficient SIRS treatments is expected to grow. The availability of advanced antibiotics and immunomodulatory therapies tailored to managing UTIs and their associated inflammation is anticipated to drive the expansion of this segment in the market.
End-User Analysis
Hospitals represent the largest end-user segment in the SIRS treatment market, holding 55.7% of the market share. This growth is expected to continue as hospitals are the primary setting for the management of systemic inflammatory response syndrome, particularly in critical care units and emergency departments. SIRS, often triggered by infections like UTIs, pneumonia, and sepsis, requires immediate intervention, and hospitals have the infrastructure, expertise, and equipment needed to provide rapid and comprehensive treatment.
The increasing incidence of sepsis, trauma, and post-surgical infections is anticipated to drive the demand for SIRS treatments within hospital settings. Additionally, hospitals are increasingly adopting advanced diagnostic and monitoring technologies that enable early identification and management of SIRS, further contributing to the growth of this segment.
As healthcare systems focus on improving patient outcomes, particularly in critical care, the role of hospitals in treating SIRS will continue to expand, ensuring that this segment remains dominant in the market. With the increasing focus on improving the quality of care in emergency and intensive care units, the demand for effective SIRS treatments in hospitals is likely to rise significantly.
Key Market Segments
By Indication
- Urinary Tract Infection (UTI)
- Autoimmune Diseases
- Pneumonia
- Others
By End-user
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Others
Drivers
High Incidence of Sepsis and Critical Illnesses is Driving the Market
The consistently high incidence of severe infections, sepsis, and other critical illnesses that often lead to Systemic Inflammatory Response Syndrome (SIRS) is a significant driver propelling the SIRS treatment market. SIRS is a common and often life-threatening condition resulting from a dysregulated inflammatory response to various insults, including infection (sepsis), trauma, burns, and pancreatitis.
Effective management of SIRS is crucial for preventing progression to organ dysfunction and reducing mortality in critically ill patients. The Centers for Disease Control and Prevention (CDC) estimates that in the US, there are approximately 1.7 million adult sepsis hospitalizations annually, with 350,000 resulting in hospital death or discharge to hospice. While SIRS is a broader syndrome that can occur without infection, these sepsis statistics underscore the immense burden of severe inflammatory responses in healthcare.
The need for early diagnosis and prompt, effective treatment to modulate this runaway inflammation is paramount to improving patient outcomes and reducing the substantial healthcare costs associated with extended hospital stays and complications. This continuous demand for interventions to manage severe inflammatory states, regardless of their underlying cause, drives the market for various SIRS treatments.
Restraints
Diagnostic Challenges and Lack of Specific Biomarkers are Restraining the Market
Significant challenges in accurately diagnosing SIRS and a persistent lack of highly specific biomarkers for its early identification are considerable restraints on the market. SIRS is defined by non-specific clinical criteria, such as alterations in temperature, heart rate, respiratory rate, and white blood cell count. These criteria are highly sensitive but lack the specificity needed to differentiate between a beneficial physiological stress response and a harmful, dysregulated inflammatory cascade that requires intervention. This ambiguity often leads to both over-diagnosis and under-diagnosis, delaying appropriate treatment or leading to unnecessary interventions.
A StatPearls review updated as recently as June 2025 emphasizes that while SIRS criteria are sensitive for early detection, they “lacked specificity” and often overlap with other conditions like trauma or pancreatitis, making it difficult to distinguish beneficial host responses from pathologic ones. The absence of a definitive, universally accepted biomarker that can precisely identify patients at risk of progressing to severe SIRS or predict their response to specific treatments limits the targeted application of therapies. This diagnostic uncertainty hinders the development and uptake of highly specific SIRS treatments, as clinicians struggle to identify the right patients at the right time, thereby restraining overall market growth.
Opportunities
Advancements in Precision Medicine and Immunomodulation are Creating Growth Opportunities
Significant advancements in precision medicine, particularly the improved understanding of inflammatory pathways and the development of targeted immunomodulatory therapies, are creating substantial growth opportunities in the SIRS treatment market. Researchers are increasingly identifying specific inflammatory mediators, genetic predispositions, and cellular mechanisms that drive dysregulated responses in SIRS. This deeper insight allows for the development of therapies that precisely target these pathways, rather than relying on broad-spectrum anti-inflammatories.
The National Institutes of Health (NIH) actively funds research into inflammatory diseases. For example, the NIH RePORT database indicates substantial funding for inflammation-related research, with a total NIH program level of US$47.311 billion in FY2024, which supports a wide range of biomedical research, including that focused on inflammatory pathways.
Furthermore, recent scientific publications, such as a March 2024 review in Frontiers in Neurology, highlight the emerging roles of specific inflammatory pathways, including the complement system, in various diseases. The ability to tailor treatments based on a patient’s individual inflammatory profile, identified through advanced diagnostics and genomic profiling, promises more effective and safer outcomes. This shift towards personalized immunomodulation represents a pivotal opportunity for novel therapies to precisely address the underlying drivers of systemic inflammation, enhancing efficacy and patient response.
Impact of Macroeconomic / Geopolitical Factors
Global macroeconomic conditions, including inflation and the overall investment in healthcare infrastructure, significantly influence the systemic inflammatory response syndrome (SIRS) treatment market by affecting drug development costs and accessibility of advanced care. Inflation can increase the expenses for pharmaceutical companies developing new SIRS therapies, impacting the cost of research, clinical trials, and manufacturing of complex biologic drugs. This may lead to higher pricing or slower market entry for novel treatments. However, the critical nature of SIRS as a life-threatening condition often prioritizes investment in treatment options regardless of economic headwinds.
According to the American Hospital Association (AHA) “Costs of Caring” report from April 2025, US hospitals faced a 5.1% increase in total expenses in 2024, with pharmaceutical costs being a significant factor, indicating persistent cost pressures within the healthcare system. Geopolitical stability is also crucial for maintaining robust global supply chains for active pharmaceutical ingredients (APIs), medical devices, and other components necessary for SIRS treatment. Despite economic fluctuations, the imperative to save lives and improve outcomes for critically ill patients ensures sustained investment in therapeutic advancements and the continuous availability of essential treatments for systemic inflammatory responses.
Evolving US trade policies, including the imposition of tariffs on imported medical devices, pharmaceuticals, and specialized components, are shaping the systemic inflammatory response syndrome (SIRS) treatment market by influencing procurement costs and supply chain resilience. SIRS treatment often involves a range of pharmaceutical products, advanced diagnostic tools, and critical care equipment, many of which rely on international supply chains for raw materials or finished products.
Tariffs on these imports can increase the financial burden on hospitals and healthcare providers, potentially leading to higher treatment costs or constraints on access to certain therapies. The American Hospital Association (AHA) reported in June 2024 that the US imported US$14.9 billion in medical equipment in 2024, highlighting the significant reliance on foreign manufacturers, with specific tariff increases on items like syringes and medical masks from certain countries taking effect.
While some medical products received tariff exclusions or extensions until May 2025, the broader policy landscape creates uncertainty. These trade policies, while aiming to bolster domestic manufacturing, can increase the operational expenses for companies producing or distributing SIRS treatments. The crucial nature of these treatments for critical care, however, often drives efforts to mitigate tariff impacts through supply chain diversification and advocacy for exemptions on life-saving medical products, ensuring continued patient access.
Latest Trends
Growing Adoption of Biomarker-Guided Therapies in Critical Care is a Recent Trend
A prominent recent trend shaping the SIRS treatment market in 2024 and continuing into 2025 is the increasing adoption of biomarker-guided therapies, particularly in critical care settings, to more precisely manage systemic inflammatory responses. Instead of broad-spectrum treatments, clinicians are increasingly utilizing specific biomarkers to stratify patients, predict disease progression, and guide therapeutic decisions, aiming for a more personalized approach to managing complex inflammatory states. This trend is driven by a greater understanding of the heterogeneity of SIRS and sepsis, recognizing that a “one-size-fits-all” approach is often ineffective.
While specific universal biomarkers for SIRS remain elusive, markers like procalcitonin for bacterial infections, and various cytokine levels are increasingly used to inform treatment strategies. A May 2025 article in PharmiWeb.com discussing precision medicine highlighted the role of data-driven approaches, including multi-omics and AI, in developing customized treatments and improving decision-making in treatment planning.
Although general precision medicine, this applies to critical care where rapid, informed decisions are crucial. This shift enables earlier intervention, helps identify patients who might benefit from specific anti-inflammatory or immunomodulatory drugs, and allows for de-escalation of therapy when inflammation resolves. The move towards more targeted, evidence-based interventions promises to optimize patient care and improve outcomes in managing severe systemic inflammation.
Regional Analysis
North America is leading the Systemic Inflammatory Response Syndrome Treatment Market
The systemic inflammatory response syndrome (SIRS) treatment market in North America, holding a significant 41.8% share, experienced notable growth in 2024. This expansion was primarily driven by the high incidence of severe infections and sepsis, which are common triggers for SIRS, alongside continuous advancements in critical care medicine and increasing healthcare expenditures.
The Centers for Disease Control and Prevention (CDC) estimates that sepsis affects at least 1.7 million Americans annually, with approximately 350,000 adults who develop sepsis in the hospital either dying or transitioning to hospice care, as of January 2025. This substantial patient burden necessitates effective treatments for the underlying inflammatory response.
Furthermore, ongoing efforts to improve early diagnosis and management of sepsis in US hospitals are contributing to the demand for advanced therapeutic interventions. The National Healthcare Safety Network annual survey revealed that 78% of US hospitals had sepsis committees in 2023, an increase from 73% in 2022, indicating a heightened focus on managing these critical conditions.
Pharmaceutical and medical device companies providing solutions for critical care and inflammatory conditions have seen sustained demand. For example, AstraZeneca reported total revenue of US$54.07 billion in 2024, an increase of US$8.45 billion from 2023, reflecting strong performance across its therapeutic areas, which include treatments for severe inflammatory and infectious diseases.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
The systemic inflammatory response syndrome treatment market in Asia Pacific is expected to grow considerably during the forecast period. This anticipated expansion is primarily fueled by the region’s large population, a rising prevalence of infectious diseases and chronic conditions that can lead to severe inflammation, and significant investments in healthcare infrastructure and critical care capabilities.
As healthcare access improves and diagnostic capabilities advance across Asia Pacific, a greater number of patients are likely to receive diagnoses and treatment for severe inflammatory conditions. The World Health Organization (WHO) highlights sepsis as a major global public health concern, affecting approximately 49 million people worldwide annually. In the Republic of Korea, the number of sepsis-related deaths in 2022 was 6,928, reflecting an increase in the mortality rate over the past decade, underscoring the ongoing challenge and the need for effective interventions.
Governments in the region are actively working to strengthen their healthcare systems and improve critical care. For instance, the World Economic Forum’s Sustainability and Resilience in Asia-Pacific Health Systems 2024 report emphasizes the region’s efforts to enhance healthcare infrastructure. Companies providing critical care solutions are projected to expand their presence.
Johnson & Johnson’s MedTech businesses, which include surgical and interventional solutions relevant to treating severe inflammatory responses, delivered over US$30 billion in sales in 2023, with continued growth across its portfolio, indicating a strong market for advanced medical technologies in the region.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the systemic inflammatory response syndrome (SIRS) treatment market are adopting various strategies to drive growth and enhance patient outcomes. They focus on developing targeted therapies, such as immunomodulators and cytokine adsorption treatments, to address the underlying inflammation in SIRS.
Companies are also investing in advanced diagnostics and biomarker identification to enable early detection and personalized treatment approaches. Strategic collaborations with academic institutions and research organizations facilitate the development of innovative therapies and expand the clinical applications of existing treatments. Additionally, market leaders are enhancing their global presence by entering emerging markets, thereby increasing accessibility to their products. Emphasis on regulatory compliance and quality assurance ensures the reliability and safety of these therapeutic solutions.
CytoSorbents Corporation, a key player in the market, specializes in blood purification technologies aimed at treating life-threatening conditions associated with excessive inflammation. Founded in 2006 and headquartered in Princeton, New Jersey, the company developed CytoSorb, a cytokine adsorbing column designed to remove harmful inflammatory mediators from the blood. This device has received CE mark approval and is utilized in critical care settings to manage patients with conditions like sepsis and SIRS. CytoSorbents continues to expand its product offerings and global reach, aiming to improve patient outcomes in critical care environments.
Top Key Players in the Systemic Inflammatory Response Syndrome Treatment Market
- Siemens Healthineers
- Recce Pharmaceuticals
- GlaxoSmithKline Plc
- F4 Pharma
- Convatec Group Plc
- AstraZeneca
- Asahi Kasei
- Adrenomed AG
Recent Developments
- In March 2024, Recce Pharmaceuticals announced the completion of cohort dosing in its Phase I/II clinical trial for RECCE 327 (R327), a treatment for urinary tract infections (UTI) and urosepsis. The trial aims to evaluate the safety, pharmacokinetics, and pharmacodynamics of various intravenous doses and infusion rates of R327.
- In November 2023, Siemens Healthineers entered into a partnership with the National Institute of Allergy and Infectious Diseases to enhance antibiotic treatments for patients suffering from sepsis.
Report Scope
Report Features Description Market Value (2024) US$ 13.3 billion Forecast Revenue (2034) US$ 24.7 billion CAGR (2025-2034) 6.4% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Indication (Urinary Tract Infection (UTI), Autoimmune Diseases, Pneumonia, and Others), By End-user (Hospitals, Ambulatory Surgical Centers, Specialty Clinics, and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Siemens Healthineers, Recce Pharmaceuticals , GlaxoSmithKline Plc, F4 Pharma, Convatec Group Plc, AstraZeneca, Asahi Kasei, Adrenomed AG. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Systemic Inflammatory Response Syndrome Treatment MarketPublished date: July 2025add_shopping_cartBuy Now get_appDownload Sample
Systemic Inflammatory Response Syndrome Treatment MarketPublished date: July 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Siemens Healthineers
- Recce Pharmaceuticals
- GlaxoSmithKline Plc
- F4 Pharma
- Convatec Group Plc
- AstraZeneca
- Asahi Kasei
- Adrenomed AG










