Regenerative Medicine Market By Therapy Type (Cell Therapy, Gene Therapy, Tissue Engineering, Small Molecule, Biologic, Progenitor, Stem Cell Therapies, Other Therapies), By Material (Synthetic Material, Biologically Derived Material, Genetically Engineered Material, Pharmaceuticals), By Application (Wound Care, Musculoskeletal, Ophthalmology, Oncology, Cardiovascular, Others), By End User (Hospitals & Clinics, Commercial Industries, and Government & Academic Research Institutes), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Sep 2025
- Report ID: 22139
- Number of Pages: 298
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
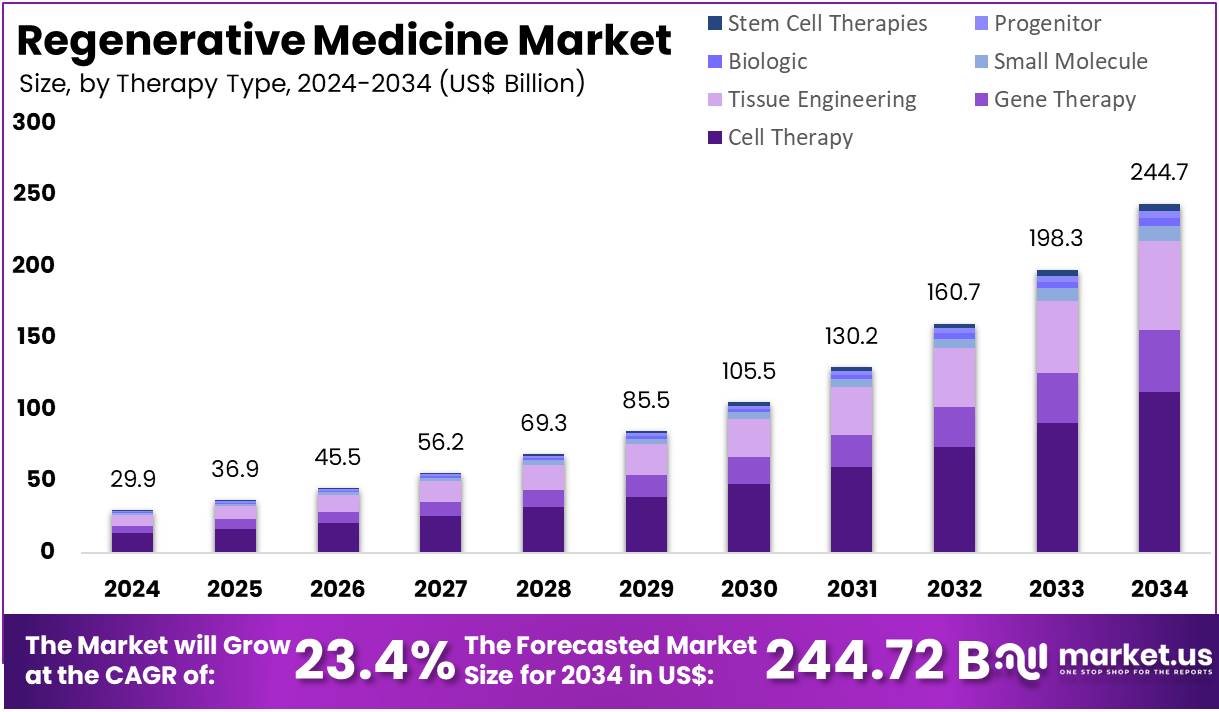
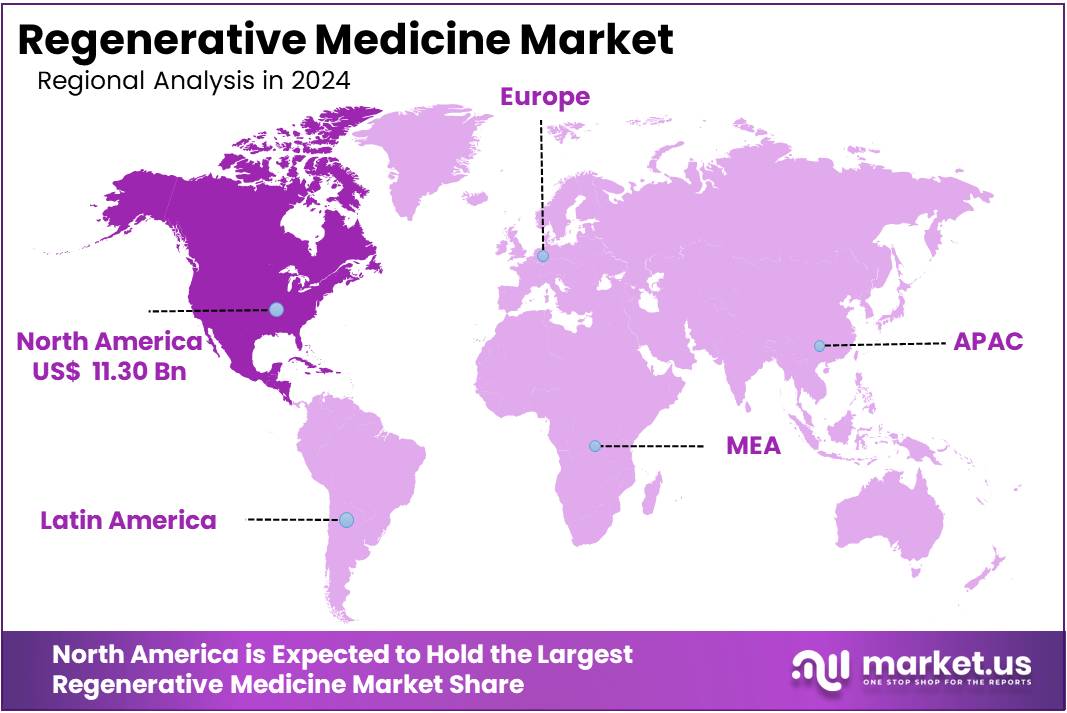
The Regenerative Medicine Market size is expected to be worth around US$ 244.72 billion by 2034 from US$ 29.89 billion in 2024, growing at a CAGR of 23.4% during the forecast period 2025 to 2034. North America held a dominant market position, capturing more than a 37.8% share and holds US$ 11.30 Billion market value for the year.
Numerous developments in biological therapies have altered the preference for personalized medical approaches over conventional treatment modalities. Market participants engaged in the development of biological therapies would benefit greatly from this. The COVID-19 epidemic has had a significant influence on several businesses, including the market for regenerative medicine.
The delivery of CAR T-cell therapy has been greatly impacted by the coronavirus outbreak. This impact has spread beyond patient care to encompass administration, logistics, and the scarcity of healthcare resources. Clinical trial enrolment and other research activity have slowed down at several universities.
Regenerative medicine focuses on repairing or replacing damaged tissues and organs using techniques like stem cell therapy, tissue engineering, and gene editing, with potential applications for diseases like diabetes and Parkinson’s. Key trends include the growing demand for personalized therapies driven by AI and genomic sequencing, advances in 3D bioprinting for organoids and tissues, development of innovative biomaterials, and integration of nanomedicine and robotics. The field is rapidly expanding, though challenges remain in regulatory processes, financing, and overcoming the immune response to engineered tissues to improve therapeutic outcomes.
In August 2025, Galapagos NV (Euronext & NASDAQ: GLPG) announced that the U.S. Food and Drug Administration (FDA) has granted RMAT designation to GLPG5101, a second-generation anti-CD19/4-1BB CAR-T therapy, for the treatment of relapsed/refractory mantle cell lymphoma (R/R MCL). The RMAT designation, introduced under the U.S. 21st Century Cures Act, is aimed at expediting the development and review of promising cell and gene therapies targeting serious or life-threatening conditions.
Key Takeaways
- In 2024, the market for Regenerative Medicine generated a revenue of US$ 29.89 billion, with a CAGR of 23.4%, and is expected to reach US$ 244.72 billion by the year 2034.
- By Therapy Type, the market is bifurcated into Cell Therapy, Gene Therapy, Tissue Engineering, Small Molecule, Biologic, Progenitor, Stem Cell Therapies, and Other Therapies with Cell Therapy taking the lead in 2024 with 46.0% market share.
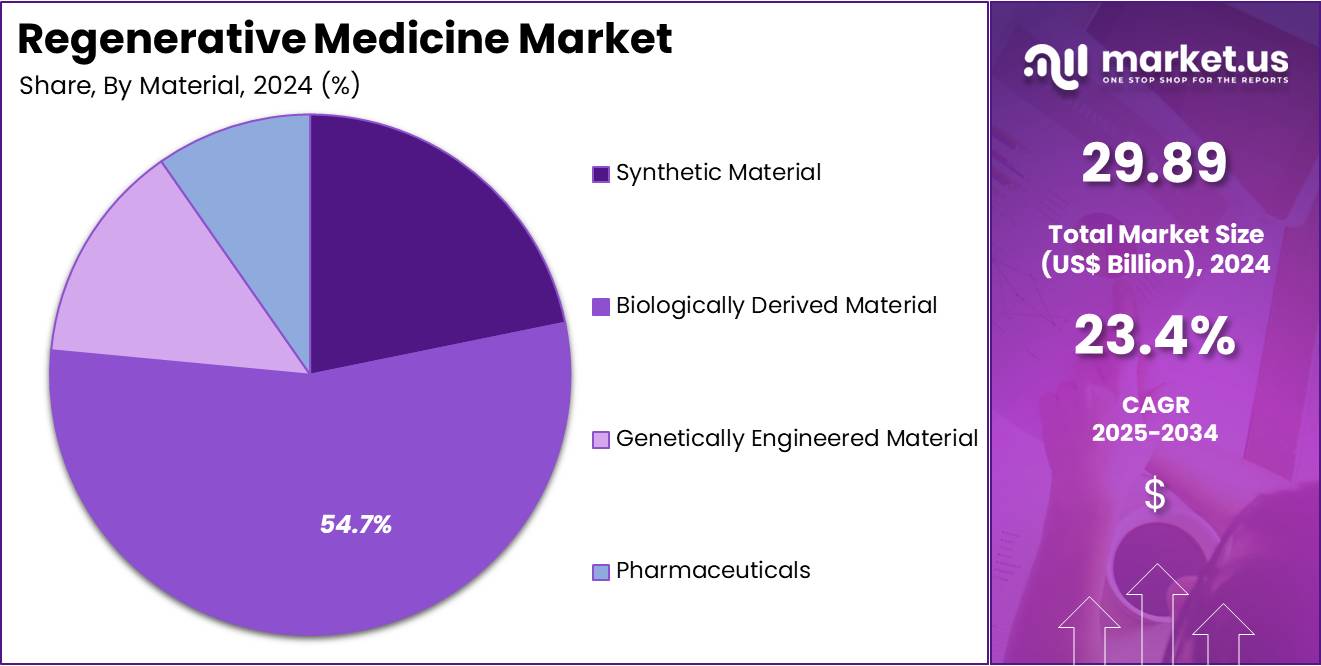
- By Material, the market is bifurcated Synthetic Material, Biologically Derived Material, Genetically Engineered Material, and Pharmaceuticals with Biologically Derived Material taking the lead in 2024 with 54.7% market share.
- By Application, the market is bifurcated into Wound Care, Musculoskeletal, Ophthalmology, Oncology, Cardiovascular, and Others with Wound Care taking the lead in 2024 with 35.6% market share.
- By End User, the market is bifurcated into Hospitals & Clinics, Commercial Industries, and Government & Academic Research Institutes with Hospitals & Clinics taking the lead in 2024 with 48.5% market share.
- North America led the market by securing a market share of 37.8% in 2024.
Therapy Type Analysis
Stem cell therapies are the dominant segment in the regenerative medicine market. Stem cells possess the unique ability to regenerate damaged tissues and organs, making them crucial in treating a wide range of diseases and conditions. These therapies are being developed for a variety of applications, including neurological disorders, heart diseases, and orthopedic injuries. For example, companies like Mesoblast Ltd. are focusing on stem cell-based treatments for cardiovascular diseases, offering new hope for patients suffering from heart failure.
The stem cell therapy segment is experiencing significant growth, driven by advancements in stem cell isolation, expansion, and differentiation technologies. Moreover, stem cells can be derived from various sources, such as bone marrow, adipose tissue, and umbilical cords, offering diverse treatment options. Stem cell therapies are anticipated to be the cornerstone of regenerative medicine, as they can potentially replace damaged cells, promote healing, and restore normal function in damaged tissues.
In January 2025, The New York Stem Cell Foundation (NYSCF) Research Institute announced a partnership with Janssen Research & Development, LLC, a Johnson & Johnson company, to utilize NYSCF’s AI-driven platform for drug discovery focused on neurodegenerative diseases. This collaboration combines NYSCF’s cutting-edge robotic systems for stem cell research with J&J’s expertise in drug discovery and data science to uncover new disease insights and speed up the development of more effective and personalized treatments.
Material Analysis
Biologically derived materials are the dominant segment in the regenerative medicine market, owing to their superior compatibility with human tissues. These materials, which include natural biomaterials such as collagen, hyaluronic acid, and fibrin, are sourced from human, animal, or microbial origins. They are used in various regenerative applications, including wound healing, tissue engineering, and drug delivery systems. Biologically derived materials promote cell adhesion, tissue regeneration, and the repair of damaged tissues, making them ideal for use in regenerative therapies. For instance, MatriStem, a biologically derived material by ACell, is used in wound care and tissue repair by providing a scaffold for cell growth.
These materials are often preferred over synthetic alternatives due to their biocompatibility, reduced immune response, and ability to integrate seamlessly with the body’s tissues. As the demand for more natural and effective solutions increases, biologically derived materials are expected to dominate the regenerative medicine market. With advancements in biotechnology and biomaterial engineering, the use of biologically derived materials in regenerative medicine is expected to expand, offering significant opportunities for market growth.
Application Analysis
Wound care is the dominant application segment in the regenerative medicine market. The global prevalence of chronic wounds, including diabetic foot ulcers, venous leg ulcers, and pressure ulcers, is a key driver for the growth of this segment. Regenerative therapies, such as stem cell-based treatments and biologically derived materials, play a crucial role in enhancing wound healing and tissue regeneration. For example, Apligraf, a biologically derived skin substitute, has been used extensively in wound care to accelerate healing and reduce complications. The growing global burden of chronic diseases like diabetes and vascular diseases is expected to increase the demand for advanced wound care treatments.
Additionally, advances in tissue engineering, which focus on developing engineered skin and other tissues for wound healing, are expanding treatment options for patients. As healthcare providers seek more effective, long-lasting solutions for wound care, regenerative therapies are anticipated to dominate this segment. With the potential to improve healing times and reduce the risk of infection and complications, wound care is expected to remain the leading application in the regenerative medicine market.
In August 2025, PolarityBio, a clinical-stage biotechnology company dedicated to addressing unmet needs in wound healing through innovative autologous regenerative skin multicellular therapy, announced its participation in the Symposium on Advanced Wound Care (SAWC) Fall 2025. The event will be held from September 3-6, 2025, in Las Vegas, Nevada.
End User Analysis
Hospitals and clinics dominate the regenerative medicine market in terms of end users. This is largely due to the high demand for advanced medical treatments in these settings, where regenerative therapies such as stem cell and gene therapies are increasingly being implemented to treat a variety of diseases. Hospitals, being primary healthcare providers, offer a wide range of services and are at the forefront of adopting innovative therapies.
For example, the use of stem cell-based treatments for joint regeneration, spinal cord injuries, and cardiovascular diseases is growing rapidly in clinical settings. Hospitals and clinics are also central to conducting clinical trials, which are crucial for the approval and adoption of new regenerative therapies. Additionally, as the market for regenerative medicine continues to expand, the infrastructure of hospitals and clinics is being upgraded to accommodate cutting-edge technologies and specialized treatments. The presence of skilled professionals and advanced facilities, such as stem cell labs and gene therapy units, further contributes to the dominance of this segment.
Key Market Segments
By Therapy Type
- Cell Therapy
- Gene Therapy
- Tissue Engineering
- Small Molecule
- Biologic
- Progenitor
- Stem Cell Therapies
- Other Therapies
By Material
- Synthetic Material
- Biologically Derived Material
- Genetically Engineered Material
- Pharmaceuticals
By Application
- Wound Care
- Musculoskeletal
- Ophthalmology
- Oncology
- Cardiovascular
- Others
By End User
- Hospitals & Clinics
- Commercial Industries
- Government & Academic Research Institutes
Drivers
Advancements in Stem Cell Technologies
One of the key drivers of the regenerative medicine market is the continuous advancements in stem cell technologies. Stem cells have the ability to differentiate into various types of cells, allowing them to replace damaged tissues or organs, offering a potential solution for a wide range of diseases and injuries that have no current treatment options. For instance, stem cell-based therapies are being developed for neurological disorders such as Parkinson’s disease and spinal cord injuries. Companies like Mesoblast Ltd. are using stem cell technology to treat cardiovascular diseases and offer new hope for patients with chronic conditions.
In June 2025, STEMCELL Technologies officially launched the STEMprep™ Tissue Dissociator System, a new benchtop instrument designed to automate, standardize, and streamline the tissue dissociation process. This system efficiently breaks down tissue samples into single-cell suspensions, enhancing research capabilities.
In addition, induced pluripotent stem cells (iPSCs) have gained significant attention due to their ability to be reprogrammed from adult cells into stem cells, overcoming ethical concerns associated with embryonic stem cells. The continued progress in stem cell technologies is expected to drive market growth as these therapies are increasingly seen as viable alternatives to traditional treatments. With breakthroughs in genetic engineering, personalized stem cell therapies are becoming a reality, and major pharmaceutical companies are investing heavily in this field. As research and clinical trials continue to yield promising results, the market for regenerative medicine is set to expand rapidly, fueled by innovations in stem cell technology.
Restraints
High Cost of Treatment
One of the primary challenges restraining the growth of the regenerative medicine market is the high cost of treatment. Regenerative therapies, especially those involving stem cells and gene editing, often require complex and expensive procedures. These therapies typically involve cutting-edge technology, highly skilled professionals, and specialized facilities. For example, CAR-T cell therapy, which involves genetically modifying a patient’s T-cells to fight cancer, costs upwards of $373,000 per treatment. Such high costs make these treatments inaccessible to many patients, especially those in low-income regions or without adequate insurance coverage.
Additionally, the need for ongoing monitoring and follow-up care further raises the financial burden for both patients and healthcare systems. The long-term affordability of regenerative treatments is also a concern, as they require extensive research and clinical testing before they can be approved for widespread use. The combination of these factors means that while regenerative medicine holds tremendous promise, the price tag remains a significant barrier to its widespread adoption. To overcome this challenge, governments and private stakeholders need to explore ways to reduce treatment costs through subsidies, insurance coverage, and cost-effective manufacturing methods. Only then can the full potential of regenerative medicine be realized on a global scale.
Opportunities
Increasing Government Funding and Investments
An emerging opportunity in the regenerative medicine market is the increasing government funding and investments being allocated to support research and development in this field. Governments around the world are recognizing the potential of regenerative medicine to address unmet medical needs, improve patient outcomes, and reduce the burden on healthcare systems. In the United States, agencies like the National Institutes of Health (NIH) and the Department of Defense have committed significant resources to stem cell and gene therapy research.
For instance, in 2020, the NIH allocated over $100 million to fund regenerative medicine projects, creating a conducive environment for innovation. Similarly, the European Union has provided funding through Horizon 2020 for regenerative medicine-related research, focusing on cell-based therapies, tissue engineering, and gene editing. In addition to direct government funding, many countries are offering tax incentives and grants to encourage private sector investments in regenerative medicine.
Moreover, In March 2025, the Honourable Anita Anand, Minister of Innovation, Science and Industry, announced a US$ 49.9 million investment through the Strategic Innovation Fund in STEMCELL Technologies Canada Inc. This funding will support the company’s US$ 222 million project to establish large-scale production of critical inputs for developing and manufacturing vaccines, therapies, and diagnostic technologies. The investment is expected to create 460 jobs and 900 four-month co-op positions for students.
This increased support enables companies to conduct clinical trials, scale up production, and bring new therapies to market faster. With more investments flowing into regenerative medicine, the sector is poised to make significant strides in treating a variety of conditions, from chronic diseases to genetic disorders, positioning it as a growing market with vast untapped potential.
Impact of Macroeconomic / Geopolitical Factors
Economic prosperity directly correlates with increased healthcare spending, fostering growth in regenerative medicine. Nations with robust economies can allocate more funds to research and development (R&D), clinical trials, and the establishment of specialized medical centers. For instance, the United States, with its substantial healthcare expenditure, has seen significant advancements in regenerative therapies, including stem cell and gene therapies.
Conversely, economic downturns can lead to budget cuts in healthcare, delaying the progression of regenerative medicine initiatives. Reduced funding may hinder the establishment of necessary infrastructure and limit access to cutting-edge treatments. Inflation impacts the cost of raw materials, labor, and manufacturing processes, leading to increased prices for regenerative therapies. This escalation can make treatments less accessible to patients and strain healthcare systems. For example, the high costs associated with stem cell therapies can limit their availability, particularly in low-income regions.
Private equity and venture capital investments play a crucial role in the development of regenerative medicine. Economic conditions that favor investment can lead to increased funding for biotech startups and established companies, accelerating innovation and market entry of new therapies. Exchange rate volatility affects the cost of importing raw materials and exporting finished products. Companies engaged in international trade may experience increased costs or reduced profit margins due to unfavorable currency movements. This can influence pricing strategies and market competitiveness in the regenerative medicine sector.
Latest Trends
Personalized Regenerative Medicine
A notable trend in the regenerative medicine market is the shift toward personalized regenerative therapies, which focus on tailoring treatments to an individual’s unique genetic makeup. This approach aims to increase the effectiveness of treatments while minimizing side effects by aligning therapies with a patient’s specific biological needs. Personalized regenerative medicine is becoming particularly significant in gene editing and cell-based therapies. Companies like CRISPR Therapeutics are leading the charge in gene editing by utilizing the CRISPR-Cas9 technology to correct genetic mutations at the DNA level, offering hope for patients with inherited diseases.
In November 2024, Cellino Biotech, Inc., a biotechnology company focused on advancing autonomous, closed biomanufacturing for personalized regenerative medicines, hosted the second annual Personalized RegenMed Forum on November 14-15, 2024, at The Engine in Cambridge, MA. The exclusive two-day event gathered scientists, industry leaders, patient advocates, and pioneers in AI and regenerative medicine from five countries to discuss the future of curative therapies.
Similarly, iPSC-based therapies are being customized to suit individual patients, as these cells can be reprogrammed to match a person’s unique genetic profile. Personalized medicine enhances the precision of treatment, offering more targeted and effective solutions for conditions like cancer, neurological disorders, and cardiovascular diseases. The increasing use of genetic testing and advanced diagnostic tools enables the identification of patients who would benefit most from personalized regenerative therapies.
Regional Analysis
North America is leading the Regenerative Medicine Market
North America is estimated to be the most lucrative market in the global regenerative medicine market, with the largest market share of 37.8%, and is expected to register a higher CAGR during the forecast period. This is due to the availability of government and private funding for research and development activities.
In June 2025, The Stem Cell Network (SCN) announced a US$13.5 million investment in 36 new regenerative medicine research projects and clinical trials. Supported by 63 partner organizations contributing over US$ 19.5 million in matching cash and in-kind support, this initiative represents a collective US$33 million boost to Canada’s regenerative medicine ecosystem. The funded projects will drive groundbreaking research across 14 disease areas, including rare diseases such as Rett syndrome and cystic fibrosis.
Asia Pacific market is expected to grow with high potential and it is expected that this region will remain growing throughout the forecast period. Europe market is having the second position in market share and revenue in the regenerative medicine market. Europe region has a high demand for organ transplant and tissue engineering.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the Regenerative Medicine market includes Pfizer Inc., Johnson & Johnson, AbbVie Inc., Amgen Inc., Medtronic Plc, Mesoblast Ltd., Fate Therapeutics, BlueRock Therapeutics, CRISPR Therapeutics, Integra LifeSciences, StemCells, Inc., Organovo Holdings, and Other key players.
Key Opinion Leaders
Leaders Opinion Dr. Sophia Williams, Chief Medical Officer at MedBio Innovations “Stem cell-based therapies are revolutionizing the treatment of degenerative diseases, offering potential to restore normal tissue function. At MedBio Innovations, we’re seeing promising results in osteoarthritis and spinal cord injury trials. The key challenge is regulatory approval and accessibility. Collaboration across biotech firms, healthcare providers, and regulators will be crucial to overcoming these barriers.” Dr. James Patterson, CEO of RegenBio Therapeutics “Gene therapy, particularly CRISPR technology, has the potential to treat genetic disorders and revolutionize regenerative medicine. However, the high cost of treatments is a major barrier. To make these therapies more accessible, we need strategic partnerships and government support. With continued investment in infrastructure and policy development, we can democratize regenerative medicine.” Dr. Emily Nguyen, Director of Research at BioStem Solutions “Biologically derived materials, like collagen, are key to accelerating tissue regeneration. At BioStem Solutions, we’re making strides in wound healing and musculoskeletal applications. The main challenge is scaling production, but with advancements in personalized medicine, biologically derived therapies will become integral to clinical practice in treating chronic diseases and injuries.” Recent Developments
- In April 2025: Atsena Therapeutics, a clinical-stage gene therapy company dedicated to reversing or preventing blindness, was reported to have received Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA) for ATSN-201, its gene therapy candidate for X-linked retinoschisis (XLRS). The therapy employs the company’s proprietary AAV.SPR spreading capsid to deliver therapeutic gene expression to central retinal photoreceptors, while avoiding surgical complications associated with foveal detachment.
- In April 2025: Fate Therapeutics, Inc. (NASDAQ: FATE), a clinical-stage biopharmaceutical company specializing in off-the-shelf cellular immunotherapies derived from induced pluripotent stem cells (iPSCs), announced that the FDA had granted RMAT designation to FT819. FT819 is an investigational iPSC-derived CAR T-cell therapy, currently in Phase 1 clinical trials, for the treatment of systemic lupus erythematosus (SLE), including patients with lupus nephritis (LN).
- In July 2024: Bioserve India launched a new portfolio of advanced stem cell products, developed by REPROCELL, for the Indian market. These products were introduced to accelerate scientific research, drug development, and regenerative medicine, thereby contributing to the advancement of therapeutic discovery in India.
Top Key Players in the Regenerative Medicine Market
- Pfizer Inc.
- Johnson & Johnson
- AbbVie Inc.
- Amgen Inc.
- Medtronic Plc
- Mesoblast Ltd.
- Fate Therapeutics
- BlueRock Therapeutics
- CRISPR Therapeutics
- Integra LifeSciences
- StemCells, Inc.
- Organovo Holdings
- Other key players
Report Scope
Report Features Description Market Value (2024) US$ 29.89 billion Forecast Revenue (2034) US$ 244.72 billion CAGR (2025-2034) 23.4% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Therapy Type (Cell Therapy, Gene Therapy, Tissue Engineering, Small Molecule, Biologic, Progenitor, Stem Cell Therapies, Other Therapies), By Material (Synthetic Material, Biologically Derived Material, Genetically Engineered Material, Pharmaceuticals), By Application (Wound Care, Musculoskeletal, Ophthalmology, Oncology, Cardiovascular, Others), By End User (Hospitals & Clinics, Commercial Industries, and Government & Academic Research Institutes) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Pfizer Inc., Johnson & Johnson, AbbVie Inc., Amgen Inc., Medtronic Plc, Mesoblast Ltd., Fate Therapeutics, BlueRock Therapeutics, CRISPR Therapeutics, Integra LifeSciences, StemCells, Inc., Organovo Holdings, and Other key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Regenerative Medicine MarketPublished date: Sep 2025add_shopping_cartBuy Now get_appDownload Sample
Regenerative Medicine MarketPublished date: Sep 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Pfizer Inc.
- Johnson & Johnson
- AbbVie Inc.
- Amgen Inc.
- Medtronic Plc
- Mesoblast Ltd.
- Fate Therapeutics
- BlueRock Therapeutics
- CRISPR Therapeutics
- Integra LifeSciences
- StemCells, Inc.
- Organovo Holdings
- Other key players










