Preeclampsia Diagnostics Market By Product Type (Consumables and Instruments), By Test Type (Urine Analysis, Blood Tests, and Others), By End-user (Hospital, Diagnostic Centers, Specialty Clinics, Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2033
- Published date: Jan 2025
- Report ID: 138346
- Number of Pages: 207
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
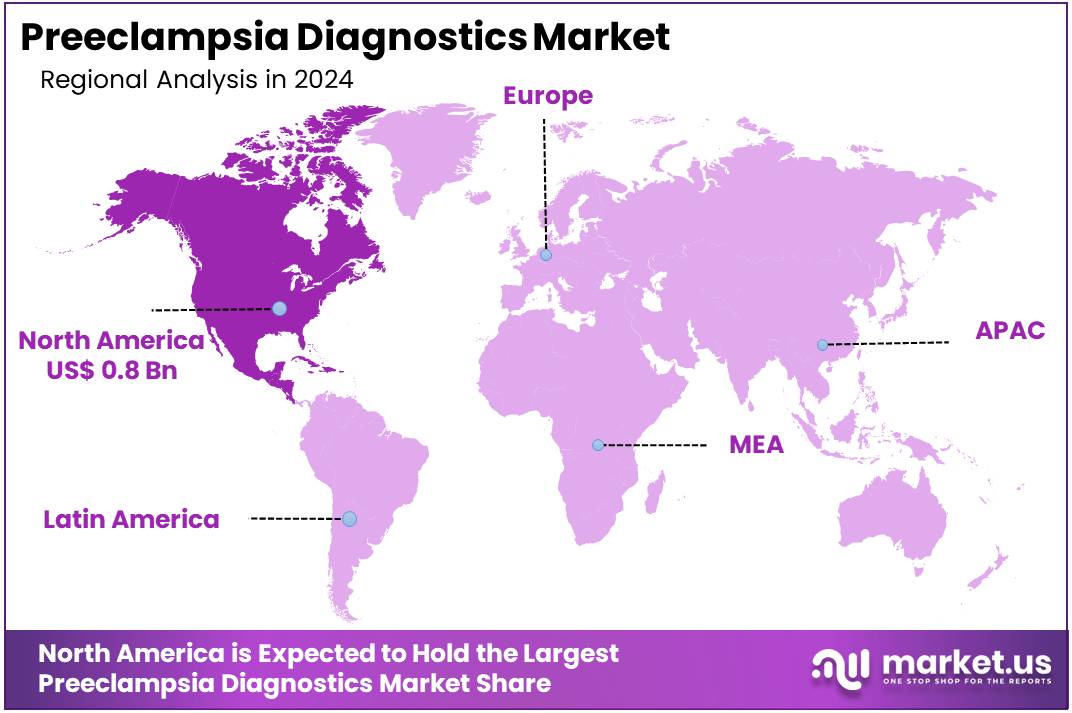
The Global Preeclampsia Diagnostics Market size is expected to be worth around US$ 5.8 Billion by 2034, from US$ 2.2 Billion in 2024, growing at a CAGR of 10.2% during the forecast period from 2025 to 2034. North America held a dominant market position, capturing more than a 38.6% share and holds US$ 0.8 Billion market value for the year.
Increasing awareness of the risks associated with preeclampsia and the need for early diagnosis are driving the growth of the preeclampsia diagnostics market. Preeclampsia, a potentially life-threatening pregnancy complication characterized by high blood pressure and organ damage, affects both the mother and the fetus, creating a significant need for timely detection and intervention.
The rising prevalence of risk factors such as obesity, hypertension, and diabetes during pregnancy is further fueling the demand for advanced diagnostic tools. In February 2024, researchers at University College Dublin began investigating preeclampsia diagnosis using technology developed by SAS, signaling the increasing role of advanced data analytics and artificial intelligence in improving diagnostic accuracy and efficiency. Recent trends show a shift towards point-of-care testing solutions that enable quick and reliable diagnosis, improving patient outcomes and reducing healthcare costs.
Additionally, the development of non-invasive biomarkers for preeclampsia offers opportunities for safer and more accessible diagnostic methods. The growing focus on personalized healthcare, where early diagnosis can guide customized treatment plans, also creates significant opportunities for the preeclampsia diagnostics market. As research progresses, innovations in diagnostic devices and testing platforms are expected to enhance detection capabilities, enabling earlier intervention and better management of the condition.

Key Takeaways
- In 2024, the market for preeclampsia diagnostics generated a revenue of US$ 2.2 billion, with a CAGR of 10.2%, and is expected to reach US$ 5.8 billion by the year 2034.
- The product type segment is divided into consumables and instruments, with consumables taking the lead in 2024 with a market share of 57.6%.
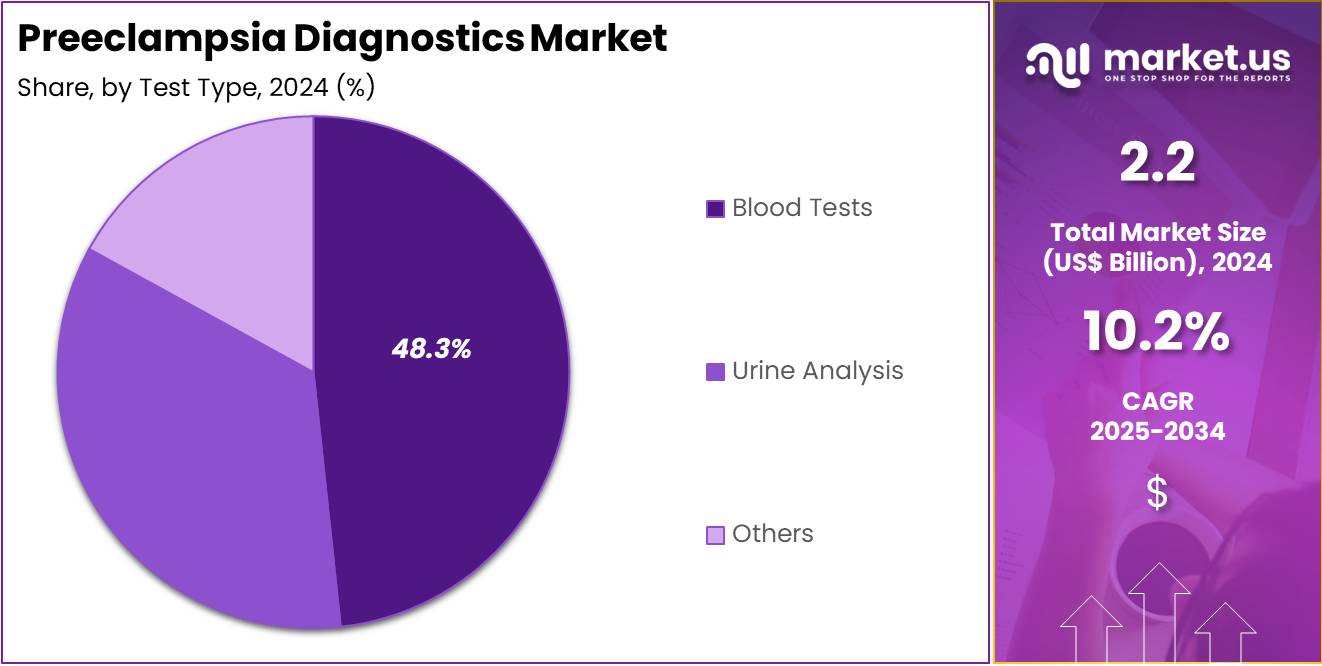
- Considering test type, the market is divided into urine analysis, blood tests, and others. Among these, blood tests held a significant share of 48.3%.
- Furthermore, concerning the end-user segment, the market is segregated into hospital, diagnostic centers, specialty clinics, others. The hospital sector stands out as the dominant player, holding the largest revenue share of 52.9% in the preeclampsia diagnostics market.
- North America led the market by securing a market share of 38.6% in 2024.
Product Type Analysis
The consumables segment led in 2024, claiming a market share of 57.6% owing to the increasing demand for reliable and accurate diagnostic tools. Consumables such as test kits, reagents, and laboratory consumables are anticipated to see rising adoption due to their essential role in routine diagnostic tests for preeclampsia.
The growing emphasis on early detection and prevention of preeclampsia in pregnant women is likely to drive the demand for consumables in diagnostic testing. Additionally, the rising number of healthcare facilities and diagnostic laboratories focused on maternal health is expected to increase the use of consumables. As technology improves and new diagnostic markers for preeclampsia are identified, the consumables segment is projected to experience continued growth in the market.
Test Type Analysis
The blood tests held a significant share of 48.3% due to the increasing preference for blood-based diagnostic methods for detecting preeclampsia. Blood tests are expected to become a crucial part of preeclampsia screening due to their ability to detect biomarkers associated with the condition, such as elevated blood pressure and abnormal protein levels.
The growing awareness of the importance of early detection in preventing complications like preterm birth and organ damage is likely to contribute to the growth of this segment. Additionally, advancements in blood testing technology, including improved sensitivity and faster results, are projected to further boost the adoption of blood tests in preeclampsia diagnostics. As healthcare providers prioritize non-invasive and accurate diagnostic options, blood tests are expected to see rising demand.
End-user Analysis
The hospital segment had a tremendous growth rate, with a revenue share of 52.9% owing to the increasing number of pregnant women seeking care in hospital settings. Hospitals are likely to remain key end-users of preeclampsia diagnostic tools, particularly in emergency and high-risk obstetric care. The growing focus on maternal health, coupled with advancements in diagnostic technology, is anticipated to increase the adoption of preeclampsia diagnostic tests in hospitals.
The need for accurate, timely detection to prevent complications such as eclampsia, organ failure, and preterm birth is projected to fuel the demand for diagnostic solutions. Additionally, as hospitals expand their maternity care services and improve patient monitoring capabilities, the hospital segment is expected to see continued growth in the preeclampsia diagnostics market.
Key Market Segments
By Product Type
- Consumables
- Instruments
By Test Type
- Urine Analysis
- Blood Tests
- Others
By End-user
- Hospital
- Diagnostic Centers
- Specialty Clinics
- Others
Drivers
Increasing Prevalence of Preeclampsia Driving the Preeclampsia Diagnostics Market
Increasing prevalence of preeclampsia is anticipated to drive the preeclampsia diagnostics market significantly. According to the World Health Organization, 5-8% of pregnancies globally are affected by preeclampsia each year, leading to approximately 76,000 maternal deaths and 500,000 fetal deaths annually. This growing health burden underscores the need for effective and early diagnostic solutions.
Advancements in diagnostic methods, including biomarkers and imaging technologies, enable timely detection and management of preeclampsia. Healthcare providers prioritize the adoption of innovative diagnostics to mitigate risks and improve maternal and fetal outcomes. Expanding awareness among pregnant women about the importance of early diagnosis fosters demand for accessible and accurate testing methods.
Research institutions and biotechnology companies increasingly collaborate to develop novel diagnostic tools tailored to high-risk pregnancies. Government initiatives aimed at reducing maternal and fetal mortality further support market growth. Integration of point-of-care testing in prenatal care enhances the accessibility and convenience of diagnostics. Technological advancements in laboratory diagnostics improve test accuracy and reduce turnaround times. These trends emphasize the critical role of diagnostic innovation in addressing the rising prevalence of preeclampsia globally.
Restraints
High Costs Are Restraining the Preeclampsia Diagnostics Market
High costs associated with preeclampsia diagnostics are restraining the market. Advanced diagnostic methods require significant investments in technology, specialized reagents, and skilled personnel, increasing the overall cost. Healthcare providers in low- and middle-income countries often face financial constraints, limiting access to cutting-edge diagnostic tools. Inadequate insurance coverage for advanced prenatal testing further discourages adoption.
Laboratories need to invest in sophisticated infrastructure to perform biomarker-based diagnostics, adding to operational expenses. Limited awareness about the benefits of early diagnostics in certain regions exacerbates cost-related challenges. The high price of reagents and consumables used in tests increases the per-patient cost, restricting widespread usage. Addressing these barriers requires cost-efficient innovations and supportive reimbursement policies to improve affordability and accessibility of preeclampsia diagnostics.
Opportunities
Increasing R&D Activities as an Opportunity for the Preeclampsia Diagnostics Market
Increasing R&D activities are anticipated to create significant opportunities for the preeclampsia diagnostics market. In September 2023, Genomics Clinical Research conducted a study exploring plasma cell-free RNA (cfRNA) as a potential diagnostic tool for preeclampsia. Such research initiatives drive the development of innovative and non-invasive diagnostic methods to improve accuracy and early detection.
Pharmaceutical and biotechnology companies increasingly invest in biomarker research to expand diagnostic capabilities. Advancements in molecular diagnostics support the identification of early-stage preeclampsia, enabling timely interventions. Collaborative efforts between academic institutions and private firms accelerate the commercialization of cutting-edge diagnostic tools.
Expanding research funding from government bodies and private organizations fosters breakthroughs in prenatal diagnostics. These innovations improve accessibility and reliability of preeclampsia testing, particularly in resource-constrained settings. These trends highlight the transformative impact of R&D in advancing diagnostic solutions and addressing maternal health challenges globally.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors play a critical role in shaping the preeclampsia diagnostics market. On the positive side, increasing healthcare investments and growing awareness of maternal health drive the demand for accurate and early diagnostic tools for preeclampsia. As the incidence of preeclampsia rises globally, governments and healthcare systems are focusing more on improving maternal care, which boosts the market.
However, economic downturns can result in reduced healthcare spending, affecting the affordability and availability of advanced diagnostic solutions. Geopolitical instability, such as trade barriers or political challenges, may disrupt the global supply chain for diagnostic equipment and reagents.
Additionally, differing regulatory standards across countries can complicate the market, especially in terms of approval and distribution. Despite these challenges, the continued advancement of diagnostic technologies and growing maternal health initiatives ensure a positive outlook for the market.
Latest Trends
Growing Awareness Driving the Preeclampsia Diagnostics Market
Increasing awareness of the risks and long-term effects of preeclampsia is driving growth in the diagnostics market. High levels of education on maternal health, particularly regarding the risks of preeclampsia and its potential long-term complications, are expected to increase demand for early screening and detection tools.
The growing focus on improving prenatal care and reducing maternal mortality rates will likely boost the adoption of advanced diagnostic methods. In August 2022, researchers from the Mayo Clinic uncovered that women who experienced severe preeclampsia exhibited more brain cell damage and higher inflammation levels than those with uncomplicated pregnancies.
This was presented at the Alzheimer’s Association International Conference, underscoring the long-term implications of preeclampsia beyond just pregnancy. As awareness continues to grow, the demand for accurate and timely diagnostic solutions is expected to rise, further driving the market’s expansion.
Regional Analysis
North America is leading the Preeclampsia Diagnostics Market
North America dominated the market with the highest revenue share of 38.6% owing to advancements in early detection technologies, increasing awareness of the condition, and the rising prevalence of high-risk pregnancies. Preeclampsia is a serious pregnancy complication, and early diagnosis is crucial for managing maternal and fetal health.
A key development in this market was the launch of an FDA-approved blood test by Thermo Fisher Scientific in July 2023, which accurately predicts preeclampsia within two weeks, boasting a 96% success rate. This innovative test has been widely praised, including by experts such as Dr. Douglas Woelkers of the University of California, San Diego, as a significant advancement in preeclampsia diagnostics. The ability to identify preeclampsia early provides physicians with more time to intervene, reducing risks for both the mother and the baby.
Additionally, the growing demand for non-invasive, accurate diagnostic tools and the increasing focus on improving maternal healthcare have fueled the market’s expansion. As more healthcare providers adopt advanced diagnostic solutions, the preeclampsia diagnostics market in North America is expected to continue its growth trajectory.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to grow with the fastest CAGR owing to improving healthcare infrastructure, a rising awareness of maternal health, and increasing investments in diagnostic technologies. Countries such as India, China, and Japan are expected to see heightened demand for preeclampsia diagnostics due to the growing prevalence of high-risk pregnancies and the focus on reducing maternal mortality rates.
In July 2021, LifeCell Diagnostics launched a preeclampsia screening program in India, which aims to detect the sFLT-1 biomarker, enabling earlier detection and more accurate diagnosis of preeclampsia, even during the third trimester of pregnancy. This initiative is anticipated to significantly impact the market by improving diagnostic accuracy and empowering healthcare professionals to make timely interventions.
Additionally, government support for maternal healthcare and the increasing adoption of advanced diagnostic technologies are likely to drive market growth in the region. As awareness of preeclampsia increases and more diagnostic solutions become available, the preeclampsia diagnostics market in Asia Pacific is expected to expand rapidly in the coming years.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the preeclampsia diagnostics market focus on developing advanced biomarker-based tests to enable early and accurate detection, improving maternal and fetal outcomes. Companies invest in R&D to create point-of-care diagnostic solutions that are easy to use and accessible in diverse healthcare settings.
Collaborations with hospitals and healthcare providers drive the adoption of innovative diagnostic tools and expand their customer base. Geographic expansion into regions with increasing awareness of maternal health strengthens market reach. Many players also emphasize regulatory compliance and cost-effectiveness to ensure widespread adoption.
Thermo Fisher Scientific Inc. is a leading company in this market, offering advanced diagnostic solutions such as biomarker assays for early detection of preeclampsia. The company combines cutting-edge research with a robust global distribution network to deliver reliable diagnostic tools. Thermo Fisher’s commitment to innovation and quality positions it as a key player in advancing maternal health diagnostics.
Top Key Players in the Preeclampsia Diagnostics Market
- Thermo Fisher Giving Inc
- Siemens Healthineers
- Roche Diagnostic
- PerkinElmer
- Metabolomic Diagnostics Ltd
- Hoffmann-La Roche Ltd
- bioMérieux
- Becton, Dickinson and Company (BD)
- Beckman Coulter
- Abbott Laboratories
Recent Developments
- In January 2024, Labcorp, a leading provider of laboratory services, introduced a US FDA-cleared blood test designed to assess and manage the risk of severe preeclampsia. Developed by Thermo Fisher Scientific, the test evaluates two biomarkers linked to preeclampsia and was recognized as one of TIME Magazine’s Best Inventions of 2023.
- In May 2023, CSEM, a renowned technology innovation center, partnered with MOMM Diagnostics, a company focused on rapid preeclampsia detection, to develop an innovative point-of-care diagnostic solution. This cutting-edge device identifies two critical biomarkers associated with preeclampsia, offering a groundbreaking approach for diagnosing this pregnancy-related condition more efficiently.
Report Scope
Report Features Description Market Value (2024) US$ 2.2 billion Forecast Revenue (2034) US$ 5.8 billion CAGR (2025-2034) 10.2% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Consumables and Instruments), By Test Type (Urine Analysis, Blood Tests, and Others), By End-user (Hospital, Diagnostic Centers, Specialty Clinics, Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Thermo Fisher Giving Inc, Siemens Healthineers, Roche Diagnostic, PerkinElmer, Metabolomic Diagnostics Ltd, F. Hoffmann-La Roche Ltd, bioMérieux, Becton, Dickinson and Company (BD), Beckman Coulter, Abbott Laboratories. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Preeclampsia Diagnostics MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample
Preeclampsia Diagnostics MarketPublished date: Jan 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Thermo Fisher Giving Inc
- Siemens Healthineers
- Roche Diagnostic
- PerkinElmer
- Metabolomic Diagnostics Ltd
- Hoffmann-La Roche Ltd
- bioMérieux
- Becton, Dickinson and Company (BD)
- Beckman Coulter
- Abbott Laboratories










