Global MRI Compatible Iv Infusion Pumps Market By Product (Device Systems, Non-magnetic pump systems, Magnetic pump system with shielding, Tubing and Disposables) By End User-(Hospitals, Ambulatory Surgical Centers, Diagnostics Imaging Centers) By Region, And Key Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends And Forecast 2024-2033
- Published date: Dec 2023
- Report ID: 28040
- Number of Pages: 222
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
The Global MRI Compatible IV Infusion Pumps Market size is expected to be worth around USD 663.2 Million by 2033 from USD 258.0 Million in 2023, growing at a CAGR of 9.9% during the forecast period from 2024 to 2033.
Magnetic Resonance Imaging (MRI) is a widely-used medical imaging technology to visualize vital organs, bodily functions, as well as to identify blockages, abnormalities, and unwanted tissue growth. Generally considered safer than other scanning techniques, MRIs are finding new applications in cardiac stress testing, intra-operative MRI surgeries, and neurology. However, the powerful magnets used in MRI scanners can present certain challenges for medical facilities and imaging centers.
Most medical devices are made with magnetic components and may not function properly in the presence of a MRI scanner. There’s also a risk that items containing ferrous metals may become projectiles due to the strong magnetic field produced by The MRI scanner, posing risks for both the patient and staff. The presence of electronic instruments nearby may also negatively affect an MRI’s imaging quality. Hospitals sometimes avoid these risks by removing patients from infusion pumps and vital signs monitors during MRI procedures.
Critically ill patients should not be removed from IV medications even for a few minutes, if removed their physical condition may deteriorate due to pain, cardiac distress, breathing difficulties, or other adverse outcomes that may go undetected by a vital signs monitor. In addition, several patients, particularly children and infants require continuous sedation to remain still during an MRI scan. The days of transferring a patient from traditional transport equipment to The MRI monitor & MRI IV pump is now a thing of the past.
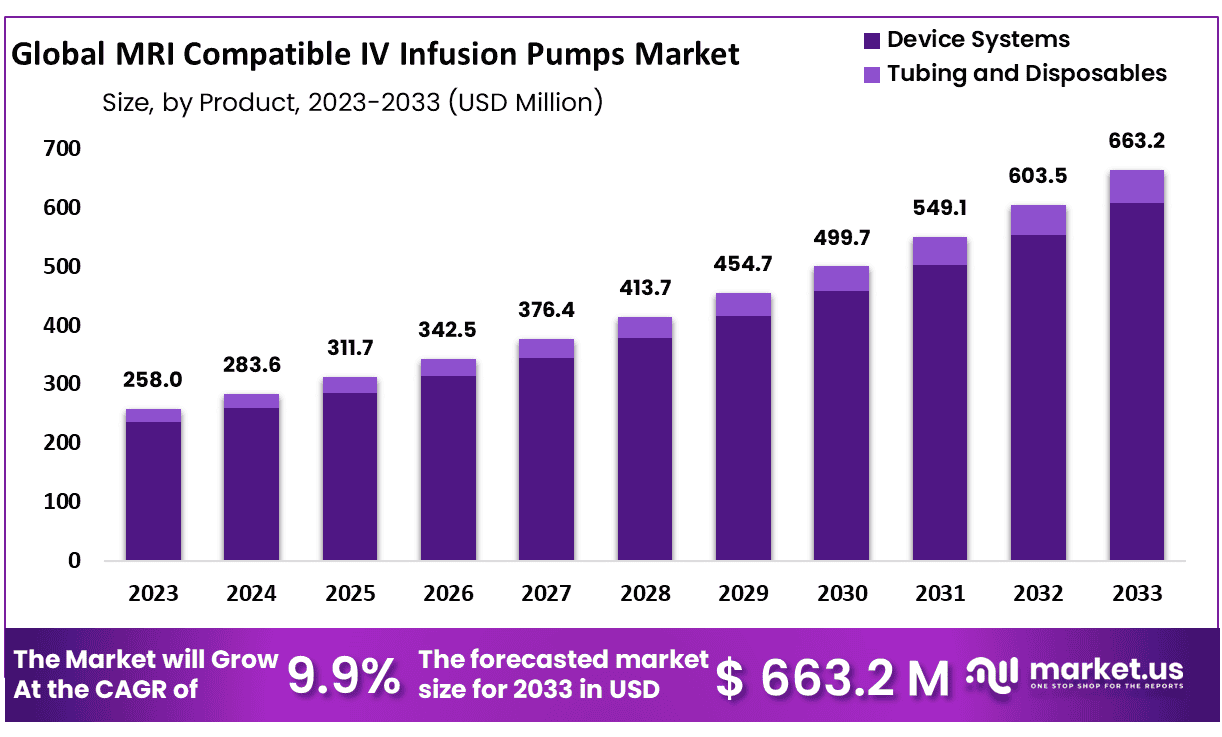
*Actual Numbers Might Vary In The Final Report
Key Takeaways
- Market Size: MRI Compatible IV Infusion Pumps Market size is expected to be worth around USD 663.2 Million by 2033 from USD 258.0 Million in 2023.
- Market Growth: The market growing at a CAGR of 9.9% during the forecast period from 2024 to 2033.
- Product Analysis: The MRI Compatible IV Infusion Pumps Market Device Systems segment commanding an impressive 91.5% market share.
- End-Use Analysis: The Hospitals segment holding 64.22% market share in 2023.
- Regional Analysis: North America accounting approx 52.3% market share and holding USD 134.9 million market revenue.
- Technological Advancements: Prominent advances in technology have resulted in the creation of MRI-compatible infusion pumps equipped with improved features that ensure patient safety and efficient drug administration during MRI scans.
- Compliance With Regulations: In this market, manufacturers must abide by stringent regulatory standards set by both the Food and Drug Administration (FDA) and other international regulating bodies to remain profitable.
- Integrating MRI Systems: Infusion pump manufacturers are prioritizing seamless integration with MRI systems to provide healthcare providers with tools that enable efficient workflows and provide efficient patient care.
Product Analysis
By product analysis, Device Systems commanding an impressive 91.5% market share. These systems play a critical role in providing intravenous infusions during magnetic resonance imaging (MRI) procedures, guaranteeing patient safety and drug delivery with complete reliability. Within this market segment, two notable subcategories can be identified as outstanding offerings: non-magnetic pump systems and magnetic pump systems with shielding. Non-magnetic pump systems are carefully engineered to operate safely within an MRI environment, giving healthcare professionals a reliable solution for infusion management. Magnetic shielding features further increase safety by protecting against interference with MRI machines.
Non-magnetic infusion pumps are small, lightweight, easy-to-use, and are designed to travel with a patient between an MRI suite and their respective care unit. These unique attributes increase an MRIs efficiency while decreasing the amount of time critically ill patients are away from their given care units.
Healthcare providers across the globe are increasingly aware of the various occupational risks that could potentially arise due to the use of magnetic MRI-compatible IV infusion pump systems. Magnetic MRI-compatible IV infusion pump systems may create safety concerns and could be harmful to patients as well as MRI operators. The magnetic components of these infusion pumps may also be responsible for variations in diagnostics and imaging.
End User Analysis
By end-user, Hospitals segment holding 64.22% market share. Medical facilities relying on magnetic resonance imaging (MRI) require IV infusion pumps compatible with magnetic resonance imaging to deliver medications safely and precisely during imaging procedures, and MRI Compatible IV Infusion Pumps help them do just that. These infusion pumps are perfect for hospital settings where accurate medication delivery is of utmost importance.
Their efficiency and compatibility with magnetic resonance imaging environments makes them essential. As demand for MRI-compatible solutions increases, more ambulatory surgical centers and diagnostic imaging centers have adopted infusion pumps as outpatient and diagnostic solutions. Hospitals play a pivotal role in improving medical imaging technology while improving patient care; hence their prevalence among infusion pumps’ users in this market.
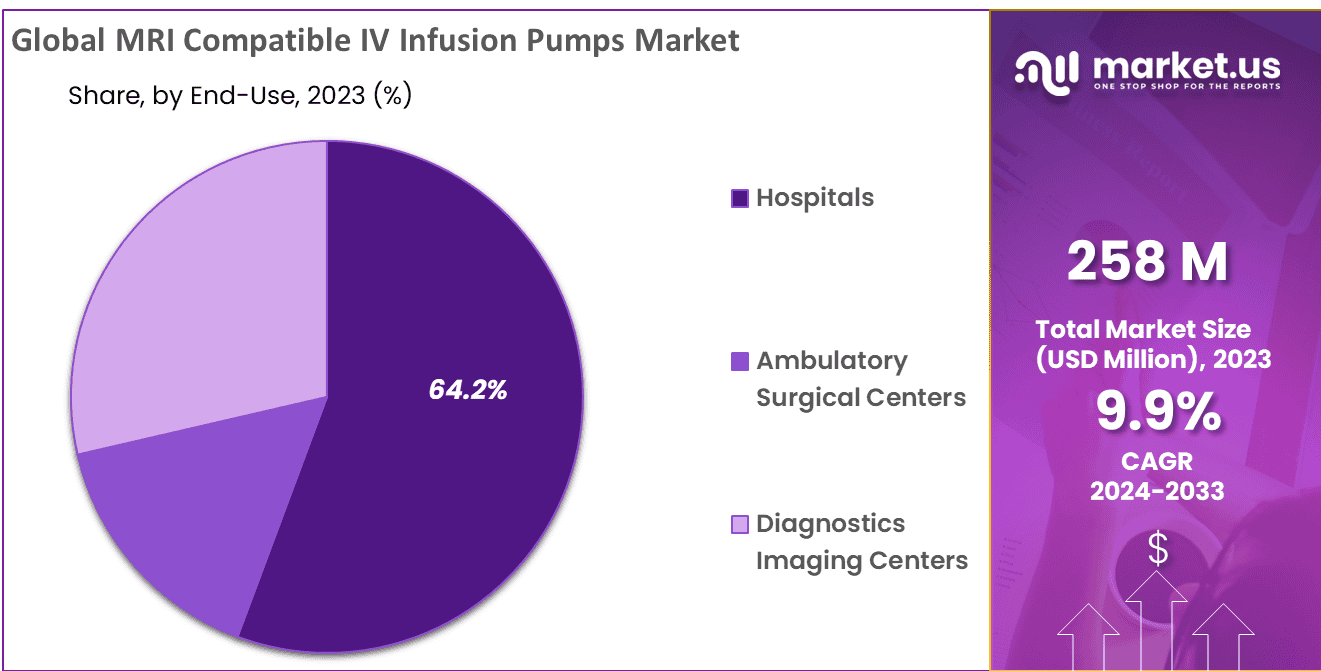
*Actual Numbers Might Vary In The Final Report
Market Segments
Product
- Device Systems
- Non-magnetic pump systems
- Magnetic pump system with shielding
- Tubing and Disposables
End User
- Hospitals
- Ambulatory Surgical Centers
- Diagnostics Imaging Centers
Driver
Rising Incidence of Chronic Illnesses
An ever-increasing global incidence of chronic illnesses that necessitate frequent MRI exams and intravenous (IV) medication administration serves as an integral driver for market expansion in MRI-Compatible IV Infusion Pump Systems market. Cancer, neurological disorders and cardiovascular conditions frequently necessitate both diagnostic imaging as well as targeted therapies delivered via IV during MRI procedures – necessitating both diagnostic imaging and targeted therapies via infusion pump systems during procedures – driving demand and market growth globally.
Technological Advancements in MRI-Compatible Systems
Recent technological developments in IV infusion pump systems designed for use within an MRI environment serve as a driving factor. Innovations such as nonmagnetic components and shielding mechanisms increase safety and efficacy within this unique environment; such innovations address the challenge of providing accurate drug delivery while safeguarding patient safety during procedures, ultimately encouraging greater adoption of such advanced systems by healthcare settings.
Trend
Increased Emphasis on Patient-Centric Designs
One trend within the market of MRI-Compatible IV Infusion Pump Systems is an increasing emphasis on user-friendly designs that cater to patient comfort during MRI procedures and minimize anxiety levels. Lightweight designs with reduced noise levels have become key trends catering to growing demands for improved patient experience and treatment plan adherence.
Integration of Connectivity and Data Management
Another notable trend is the increasing convergence between connectivity and data management features on MRI-compatible infusion pump systems and healthcare providers’ demand for seamless integration with EHRs, real-time monitoring of infusion parameters, and digital transformation within medical settings – leading to efficient data management practices and improving workflow overall. This trend fits in perfectly with this digital revolution of healthcare delivery.
Restraint
High Initial Costs and Budgetary Constraints
One major roadblock to market growth is the high initial costs associated with purchasing and installing MRI-compatible IV infusion pump systems. Their incorporation of advanced technologies, nonmagnetic materials, and safety features increases price points considerably; budgetary constraints within healthcare facilities – particularly resource-limited settings – may prevent widespread adoption, hindering their market expansion.
Complex Regulatory Compliance Requirements
Manufacturers and healthcare facilities face complex regulatory compliance requirements with difficulty. Ensuring that MRI-compatible IV infusion pump systems conform to stringent regulatory standards adds to the complexity of development and approval processes; successfully navigating these hurdles can take time and resources, slowing the speed at which new innovations reach market.
Opportunities
Expansion of MRI Facilities and Imaging Centers
The increase in global MRI facilities and imaging centers presents manufacturers with an unparalleled opportunity. As demand for diagnostic MRI procedures rises, these facilities require innovative IV infusion pump systems compatible with imaging environments – offering manufacturers an excellent chance to collaborate with healthcare providers and imaging centers by offering advanced and reliable IV infusion pump solutions that address evolving market needs for diagnostic imaging procedures.
Rising Adoption of Ambulatory Surgical Centers
The growing adoption of ambulatory surgical centers (ASCs) provides manufacturers with a market expansion opportunity. ASCs increasingly perform MRI-guided procedures, increasing demand for infusion pump systems compatible with this imaging modality in these settings. Manufacturers can capitalize on this trend by developing solutions tailored specifically for ASC environments; building partnerships with them; and expanding their presence within outpatient care.
Regional Analysis
On the basis of region, the global MRI compatible IV infusion pumps are segmented into North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. North America accounting approx 52.3% market share and holding USD 134.9 million market revenue. Among these aforementioned regions, the markets in North America are expected to account for the majority revenue share of the global MRI compatible IV infusion pumps market over the forecast period.
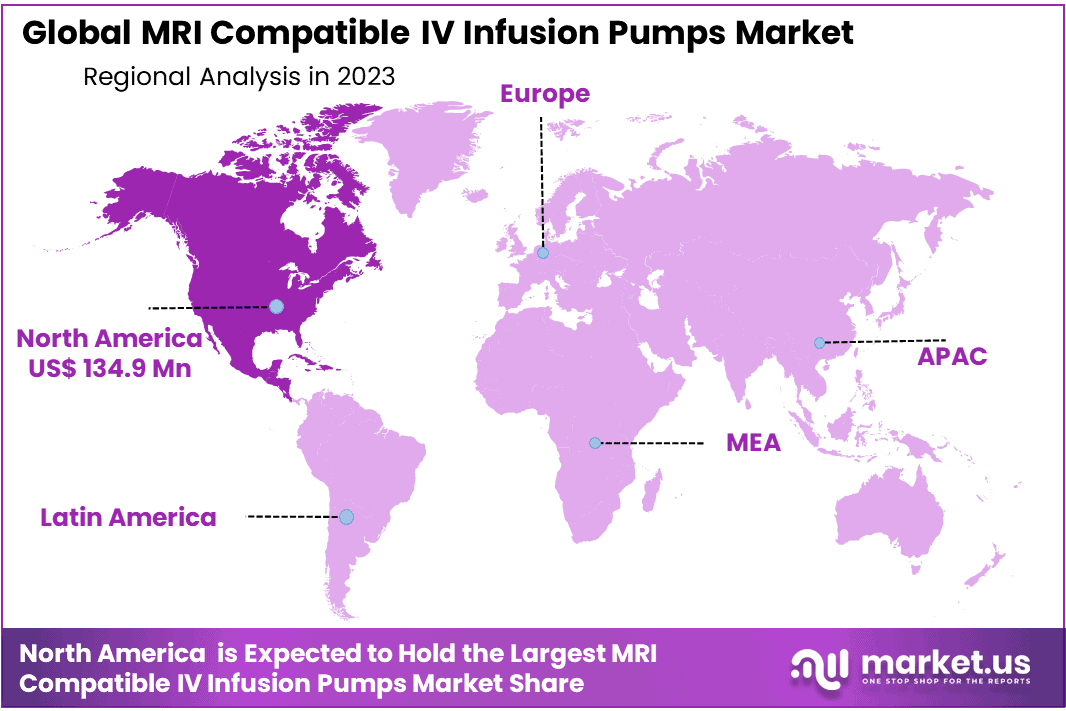
*Actual Numbers Might Vary In The Final Report
Key Regions and Countries
North America
- The US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Player Analysis
These reports comprehensively analyze notable advances in the MRI-Compatible IV Infusion Pump Systems market through both organic and inorganic growth strategies. Companies are actively engaging in organic growth initiatives such as product launches, approvals, patent applications and event participation; whilst inorganic strategies have also played a significant role in expanding business operations and broadening customer bases for key players within this market.
Market Key Players
- iRadimed Corporation
- B.Braun Melsungen AG
- Fresenius Kabi AG
- Smiths Medical (Smiths Group plc.)
- Arcomed AG
- Becton, Dickinson Company (Carefusion)
- Baxter International Inc.
Recent Developments
- iRadimed Corporation: Received FDA clearance in October 2023 for its new MRidium Horizon wireless MRI-compatible IV infusion pump system. This lightweight and portable pump offers enhanced mobility and patient comfort during scans.
- B.Braun Melsungen AG: Launched the SpacePlus+ MRI-compatible infusion pump in Europe in July 2023. This pump features a modular design for flexibility and ease of use.
- Fresenius Kabi AG: Focused on developing smart infusion pumps with advanced data analytics capabilities for improved medication safety and patient monitoring.
- Smiths Medical (Smiths Group plc.): Acquired Ivacare in September 2023, a leading provider of ambulatory infusion pumps, strengthening their presence in the home healthcare market.
- Arcomed AG: Emphasized the development of compact and lightweight MRI-compatible pumps for improved patient comfort and access to MRI scanners.
- Baxter International Inc.: Collaborating with academic institutions to evaluate the clinical and economic benefits of MRI-compatible infusion pumps in various patient populations.
Report Scope
Report Features Description Market Value (2023) USD 258.0 Million Forecast Revenue (2033) USD 663.2 Million CAGR (2024-2033) 9.9% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Product-(Device Systems, Non-magnetic pump systems, Magnetic pump system with shielding, Tubing and Disposables);By End User-(Hospitals, Ambulatory Surgical Centers, Diagnostics Imaging Centers) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape iRadimed Corporation, B.Braun Melsungen AG, Fresenius Kabi AG, Smiths Medical (Smiths Group plc.), Arcomed AG, Becton, Dickinson Company (Carefusion), Baxter International Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  MRI Compatible IV Infusion Pumps MarketPublished date: Dec 2023add_shopping_cartBuy Now get_appDownload Sample
MRI Compatible IV Infusion Pumps MarketPublished date: Dec 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- iRadimed Corporation
- B.Braun Melsungen AG
- Fresenius Kabi AG
- Smiths Medical (Smiths Group plc.)
- Arcomed AG
- Becton, Dickinson Company (Carefusion)
- Baxter International Inc.









