Global Medical Devices Vigilance Market By Delivery Mode(On-Demand, On-Premise) By Application(Diagnostics, Therapeutics, Surgical, Research) By End-User(Clinical Research Organizations (CROs), Business Process Outsourcing (BPO), Original Equipment Manufacturers (OEM), Other End-Users) and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Jan 2024
- Report ID: 84635
- Number of Pages: 318
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
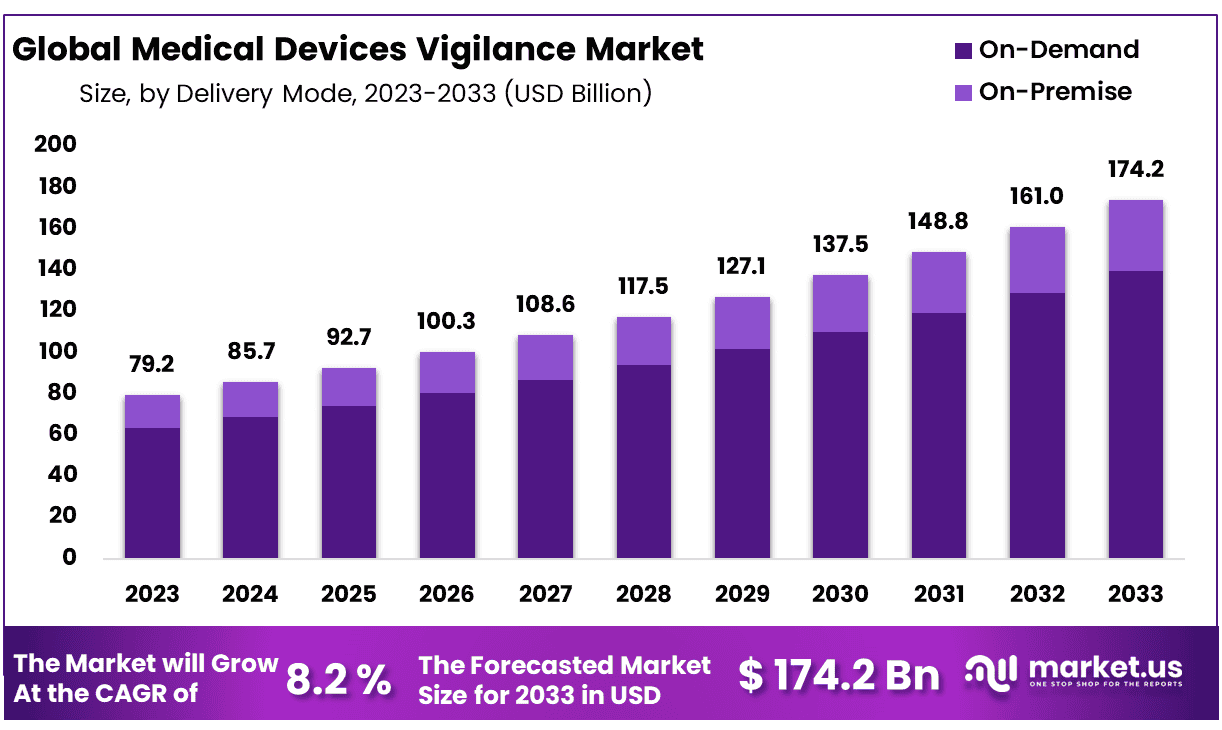
The Global Medical Devices Vigilance Market size is expected to be worth around USD 174.2 Billion by 2033 from USD 79.2 Billion in 2023, growing at a CAGR of 8.2% during the forecast period from 2024 to 2033.
Medical devices span a wide range of products, from diagnostic medical devices used to detect diseases and anomalies to therapeutic medical devices used to cut tissue, cover wounds, or close open clogged arteries, to extremely complicated and computerized medical equipment. Because of their diversity and the crucial necessity for treatment and patient care, it is becoming increasingly important to regulate the manufacturing, operating, and distribution processes to assure their quality, safety, and efficiency.
The collection, evaluation, reporting, quality check, and recognition of the condition of medical devices as a result of their use is known as medical device vigilance or materiovigilance. Medical device vigilance attempts to protect patients, healthcare facilities that use medical devices, and others by preventing or reducing the occurrence of medical device-related incidents. Medical device vigilance monitors the detection of technical faults and potential adverse effects linked to medical equipment.
Medical devices such as prosthetic limbs and artificial organs are often state-of-the-art and can be very expensive. Consequently, these devices’ safety is vital to patient safety. The FDA monitors any device considered “high risk,” such as medical devices implanted in the human body and drug delivery systems, and requires manufacturers to register these products with the FDA. Medical devices are very important to patient care, with over 20% of hospital expenditures being spent on them. Patients may use these devices for weeks or years, but they are often left untested for safety and efficacy before coming into the market or being adopted by hospitals. The FDA does not have enough resources to keep up with the ever-growing number of new devices, leaving their safety to patients’ trust in manufacturers.
Key Takeaway
- Market Size: Medical Devices Vigilance Market size is expected to be worth around USD 174.2 Billion by 2033 from USD 79.2 Billion in 2023.
- Market Growth: The market growing at a CAGR of 8.2% during the forecast period from 2024 to 2033.
- Delivery Mode Analysis: The On-Demand delivery mode stands out as the dominant force, commanding a remarkable 80% share of the market.
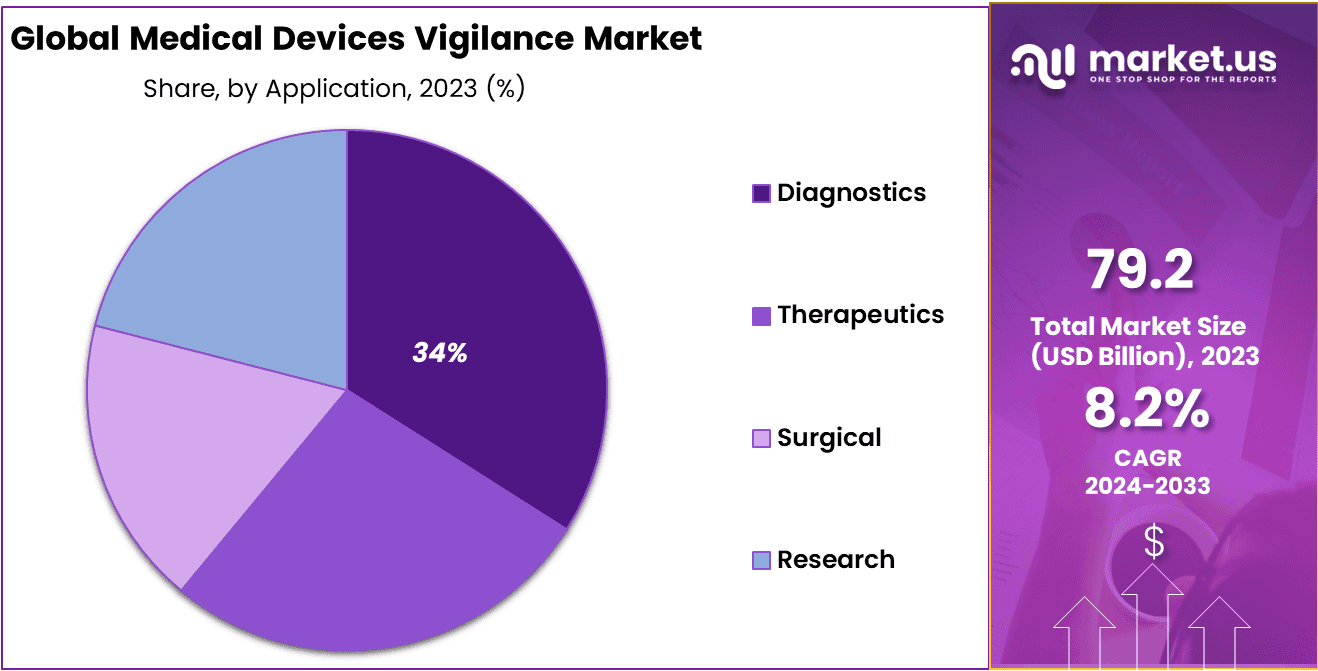
- Application Analysis: Diagnostics holds a substantial market share, accounting for 34% of the total market.
- End-Use Analysis: Clinical Research Organizations (CROs) stand out as the dominant force, commanding a substantial 40% share of the market.
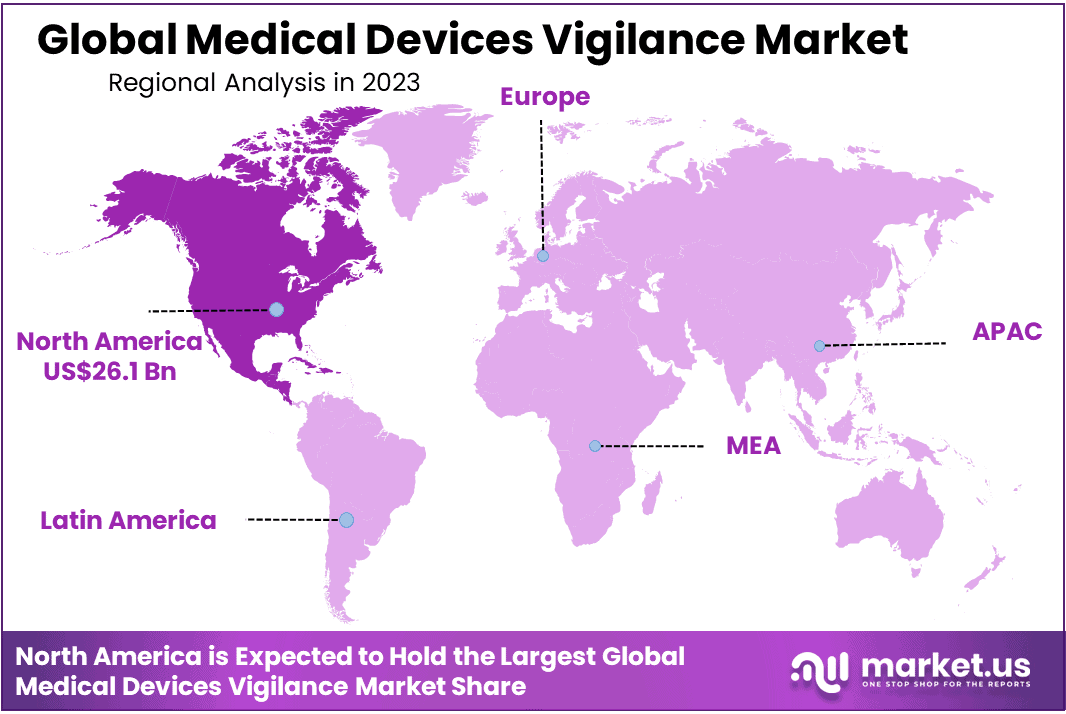
- Regional Analysis: North America commands a significant market share of 33%, generating a substantial market revenue of USD 26.1 billion.
Delivery Mode Analysis
In the Medical Devices Vigilance Market, the delivery mode landscape is characterized by two primary models: On-Demand and On-Premise. Among these, the On-Demand delivery mode stands out as the dominant force, commanding a remarkable 80% share of the market.
On-Demand Medical Devices Vigilance solutions offer several advantages that contribute to their widespread adoption. They provide real-time access to data and analytics, enabling healthcare organizations to promptly identify and respond to adverse events and safety concerns related to medical devices. Moreover, On-Demand solutions often come with the benefit of scalability and flexibility, allowing healthcare providers to adjust their usage based on evolving needs without significant infrastructure investments.
On the other hand, the On-Premise delivery mode, while still significant, faces competition from the convenience and accessibility offered by On-Demand solutions. On-Premise solutions involve the installation and maintenance of software within the organization’s infrastructure, which can be costlier and less flexible in adapting to changing requirements.
Application Analysis
In the dynamic landscape of the Medical Devices Vigilance Market, different sectors play a crucial role in shaping the industry. Among these sectors, Diagnostics holds a substantial market share, accounting for 34% of the total market.
Diagnostics in the Medical Devices Vigilance Market involve the monitoring, reporting, and analysis of data related to diagnostic medical devices. This sector is pivotal in ensuring the safety and effectiveness of diagnostic tools, such as imaging devices, in vitro diagnostics, and diagnostic software. With an increasing emphasis on early disease detection and accurate diagnostics, the demand for vigilant oversight in this segment is on the rise.
In addition to Diagnostics, other sectors such as Therapeutics, Surgical, and Research also contribute significantly to the overall market. Each of these sectors has its unique set of challenges and safety considerations. The Therapeutics segment involves vigilance related to medical devices used in treatment, while the Surgical sector deals with devices used in surgical procedures. Research encompasses devices utilized in clinical trials and research settings.
End User Analysis
In the realm of the Medical Devices Vigilance Market, different end-user segments contribute significantly to its growth and evolution. Notably, Clinical Research Organizations (CROs) stand out as the dominant force, commanding a substantial 40% share of the market.
Clinical Research Organizations (CROs) play a pivotal role in medical device vigilance by conducting comprehensive research, clinical trials, and post-market surveillance to ensure the safety and efficacy of these devices. Their expertise in regulatory compliance and data management positions them as key stakeholders in the vigilance process, making them indispensable in the industry.
In addition to CROs, other essential end-user segments include Business Process Outsourcing (BPO) firms and Original Equipment Manufacturers (OEMs). BPO firms provide outsourced services related to data management, adverse event reporting, and regulatory compliance, offering cost-effective solutions to healthcare organizations. OEMs, on the other hand, are the manufacturers of medical devices themselves, making their active involvement in vigilance critical for ensuring product safety and regulatory compliance.
Market Segments
Delivery Mode
- On-Demand
- On-Premise
Application
- Diagnostics
- Therapeutics
- Surgical
- Research
End-User
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-Users
Drivers
Stringent Regulatory Requirements
The Medical Devices Vigilance Market is primarily driven by the increasing regulatory scrutiny and requirements imposed by health authorities worldwide. As governments and regulatory bodies demand higher safety standards for medical devices, manufacturers are compelled to invest in vigilance systems to ensure compliance and reduce the risk of costly recalls and legal consequences.
Rising Medical Device Usage
With the global population aging and the increasing prevalence of chronic diseases, there is a growing demand for medical devices. This surge in usage, ranging from diagnostic equipment to implantable devices, is a significant driver of the vigilance market. The more devices in circulation, the greater the need for robust vigilance systems to monitor their safety and performance.
Trends
Advanced-Data Analytics
One notable trend in the Medical Devices Vigilance Market is the adoption of advanced data analytics and artificial intelligence (AI) tools. These technologies enable faster and more accurate analysis of adverse event reports, helping companies identify potential safety issues early and take proactive measures.
The Office of Medical Devices Vigilance (OMDV) is a branch of the FDA that conducts investigations to ensure the safety and efficacy of medical devices. The FDA relies on patient reports, hospital reports, injury or event reports, and submitted samples when investigating medical devices. A submitted sample could be anything from blood to skin samples. OMDV also investigates audits made by manufacturers when questions arise about the safety or efficacy of their products.
Global Harmonization
The industry is witnessing a trend toward global harmonization of vigilance reporting standards. This simplifies compliance for multinational companies, reduces duplication of efforts, and enhances the exchange of safety information across borders. The adoption of the International Medical Device Regulators Forum (IMDRF) framework is a prime example of this trend.
Restraints
Resource Intensiveness
Implementing and maintaining a robust vigilance system can be resource-intensive for medical device manufacturers. The collection, analysis, and reporting of adverse events require skilled personnel and technology investments. Small to mid-sized companies may face challenges in allocating sufficient resources for this purpose.
Data Privacy Concerns
As vigilance systems collect and analyze patient data, privacy concerns become a significant restraint. Ensuring compliance with data protection regulations, such as GDPR in Europe, adds complexity and costs to vigilance activities.
Opportunities
Market Expansion
The Medical Devices Vigilance Market presents opportunities for service providers to expand their offerings. Customized vigilance solutions, consultancy services, and data analytics expertise are in demand as companies seek comprehensive support in meeting regulatory requirements.
Technological Advancements
Continued advancements in technology, such as the Internet of Things (IoT) and wearable medical devices, create new opportunities for vigilance. These innovations enable real-time monitoring and quicker detection of safety issues, enhancing patient outcomes and reducing risks.
Regional Analysis
North America commands a significant market share of 33%, generating a substantial market revenue of USD 26.1 billion. This dominance is attributed to the escalating number of reported adverse events, which is driving the adoption of vigilance systems in the region.
also, the Asia-Pacific region is poised for the highest growth rate during the forecast period from 2024 to 2033. This growth is underpinned by a large and diverse patient population and a burgeoning trend of clinical research outsourcing within the region.
Within the medical devices vigilance market report, the country-specific section furnishes crucial insights into factors impacting individual markets and domestic regulatory changes that influence both current and future market trends.
Key Regions and Countries
North America
- The US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Market Player Analysis
Prominent players in the medical devices vigilance market are primarily concentrating their efforts on the development of both software and devices. These industry leaders are also executing strategic initiatives, such as forming partnerships and engaging in acquisitions, to fortify their presence within the global medical devices vigilance market. For example, AB-Cube introduced Safety Easy MD, a medical device vigilance software tailored for managing adverse events.
The primary objective behind introducing this software is twofold: to broaden its customer base and bolster its position in the overall medical devices vigilance market. Furthermore, major manufacturers in the medical devices vigilance sector are making substantial investments in research and development endeavors to diversify their product offerings and expand their customer reach, particularly in emerging regions.
Market Key Players
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical
- Omnify Software, Inc.
- ZEINCRO
- AssurX, Inc.
Recent Developments
- Sparta Systems: Launched TrackWise RTM, a cloud-based platform for medical device traceability and regulatory compliance.
- Oracle Corporation: Integrated Oracle Health Sciences Data Management Platform with Oracle Argus Safety Suite for streamlined vigilance data management.
- Xybion Corporation: Released Xybion Vigilance 10.5 with enhanced functionalities for data capture, risk identification, and reporting.
- Sarjen Systems Pvt. Ltd: Developed VigilanceOne, a comprehensive vigilance software platform for medical device manufacturers in India.
- MDI Consultants, Inc.: Launched new consulting services for post-market surveillance and quality management systems in medical devices.
Report Scope
Report Features Description Market Value (2023) USD 79.2 Billion Forecast Revenue (2033) USD 174.2 Billion CAGR (2024-2033) 8.2% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Delivery Mode-(On-Demand, On-Premise);By Application-(Diagnostics, Therapeutics, Surgical, Research);By End-User-(Clinical Research Organizations (CROs), Business Process Outsourcing (BPO), Original Equipment Manufacturers (OEM), Other End-Users) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Sparta Systems, Oracle Corporation, Xybion Corporation, Sarjen Systems Pvt. Ltd, MDI Consultants, Inc., AB-Cube, Laerdal Medical, Omnify Software, Inc., ZEINCRO, AssurX, Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the Medical Devices Vigilance Market?The Medical Devices Vigilance Market is a sector focused on monitoring and ensuring the safety of medical devices to detect and manage adverse events or risks associated with them.
How big is the Medical Devices Vigilance Market?The global Medical Devices Vigilance Market size was estimated at USD 79.2 Billion in 2023 and is expected to reach USD 174.2 Billion in 2033.
What is the Medical Devices Vigilance Market growth?The global Medical Devices Vigilance Market is expected to grow at a compound annual growth rate of 8.2%. From 2023 To 2033
Who are the key companies/players in the Medical Devices Vigilance Market?Some of the key players in the Medical Devices Vigilance Markets are Sparta Systems, Oracle Corporation, Xybion Corporation, Sarjen Systems Pvt. Ltd, MDI Consultants, Inc., AB-Cube, Laerdal Medical, Omnify Software, Inc., ZEINCRO, AssurX, Inc.
Why is vigilance important in the medical devices industry?Vigilance is crucial in the medical devices industry to protect patient safety, identify potential issues with devices, and ensure regulatory compliance.
How can vigilance software benefit companies?Vigilance software helps companies track adverse events, enhance customer base expansion, and strengthen their position in the market.
Why are manufacturers investing in research and development?Manufacturers invest in R&D to diversify their product portfolio and reach new customers, especially in emerging regions.
 Medical Devices Vigilance MarketPublished date: Jan 2024add_shopping_cartBuy Now get_appDownload Sample
Medical Devices Vigilance MarketPublished date: Jan 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical
- Omnify Software, Inc.
- ZEINCRO
- AssurX, Inc.










