Materiovigilance Market Analysis By Delivery Mode (On-premise, Cloud-Based), By Application (Diagnostic Application, Therapeutic Application, Surgical Application, Research Application, Others), By End Users (Contract Research Organization, Business Process Outsourcing, Original Equipment Manufacturers, Others), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Nov 2024
- Report ID: 84594
- Number of Pages: 209
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
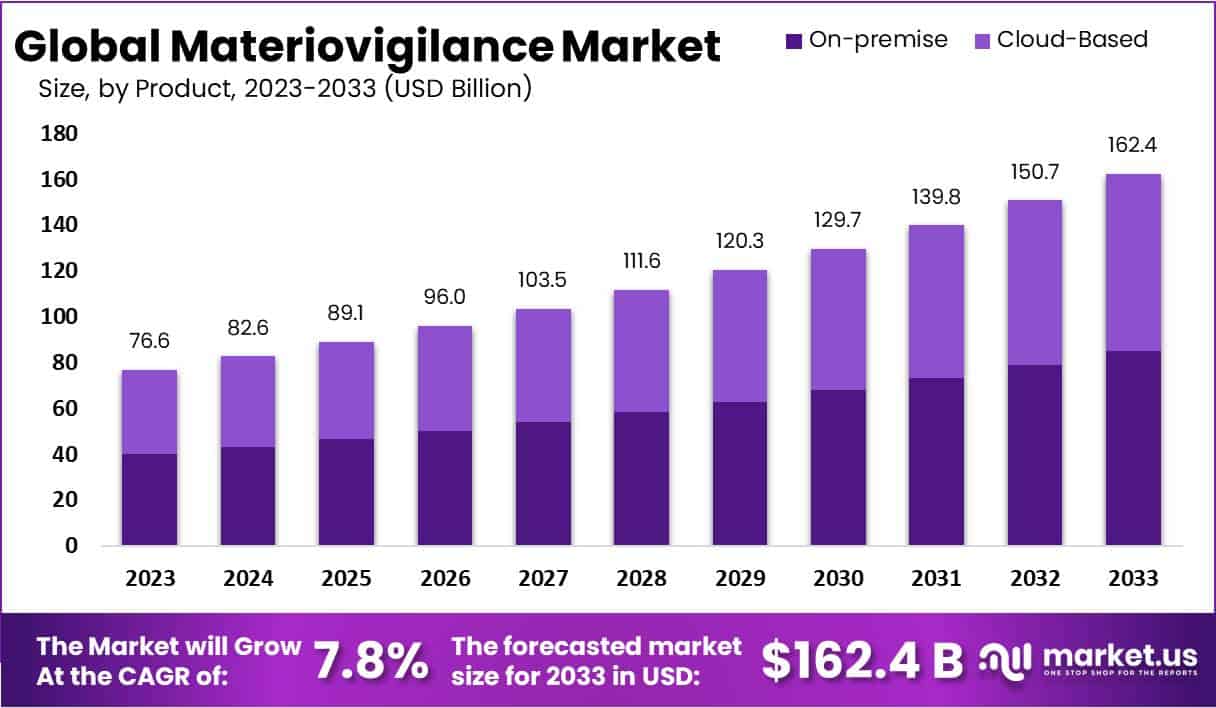
The Global Materiovigilance Market size is expected to be worth around US$ 162.4 Billion by 2033, from US$ 76.6 Billion in 2023, growing at a CAGR of 7.8% during the forecast period from 2024 to 2033.
Materiovigilance refers to the science and activities related to the surveillance, assessment, and reporting of adverse events or incidents associated with medical devices. This field is analogous to pharmacovigilance, which deals with monitoring and ensuring the safety of pharmaceutical products. Materiovigilance is crucial for identifying and addressing potential risks and issues associated with the use of medical devices to enhance patient safety.
Key activities in materiovigilance include the collection and analysis of data on adverse events, monitoring of device performance, and implementation of measures to mitigate risks. Materiovigilance programs are typically established by regulatory authorities and healthcare organizations to monitor the safety and effectiveness of medical devices throughout their lifecycle.
The Materiovigilance Market refers to the business and economic aspects associated with the materiovigilance activities. This market encompasses the services, technologies, and solutions that support the monitoring and reporting of adverse events related to medical devices. Key stakeholders in the materiovigilance market include regulatory bodies, healthcare providers, medical device manufacturers, and technology vendors.
Factors driving the materiovigilance market include increasing regulatory requirements for medical device safety, the growing complexity of medical devices, and the rising awareness of the importance of monitoring and reporting adverse events. The market may include software solutions for data management, consulting services, training programs, and other resources aimed at improving materiovigilance practices.
Key Takeaways
- The Materiovigilance Market is expected to expand at a 7.8% CAGR, potentially reaching US$ 162.4 billion by 2033 from US$ 76.6 billion in 2023.
- On-premise delivery modes led in 2023, holding a 52.3% market share, noted for their reliability and localized control features.
- Cloud-Based delivery modes, known for their flexibility and scalability, were slightly behind in market share in 2023.
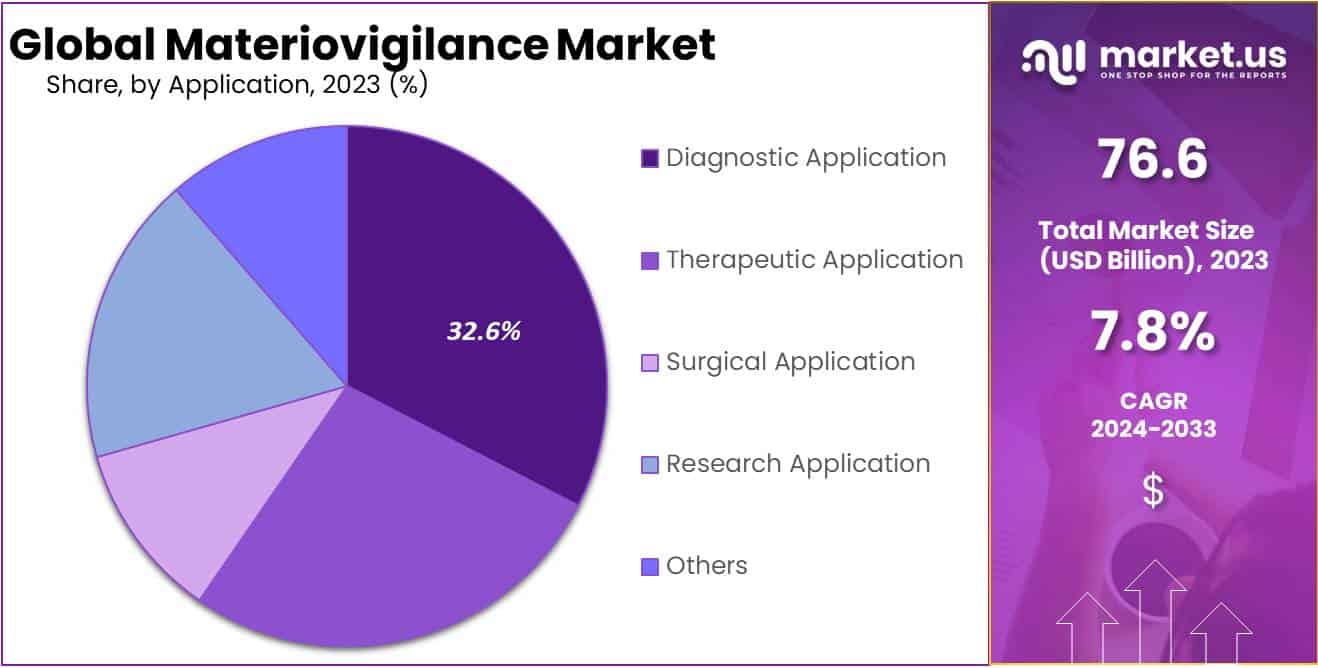
- Diagnostic applications dominated the market with a 32.6% share in 2023, emphasizing the safety of diagnostic materials.
- Other significant market contributions came from therapeutic, surgical, and research applications.
- Original Equipment Manufacturers (OEM) maintained a leading position with a 46.8% market share among end users in 2023.
- Contract Research Organizations (CROs) and Business Process Outsourcing (BPO) sectors also significantly influenced the market.
- Major growth drivers include stringent regulatory standards and an increase in adverse event reports.
- North America was the leading region with a 46.2% market share and a value of USD 35.3 billion in 2023.
- Potential growth opportunities are seen in the rising adoption of Cloud-Based solutions, untapped markets, and strategic healthcare collaborations.
Delivery Mode Analysis
In 2023, the Materiovigilance market showcased a robust landscape, with the On-premise delivery mode emerging as the frontrunner, securing a dominant market position by capturing over 52.3% share. This segment’s supremacy can be attributed to its inherent reliability and familiarity, providing users with a tangible, dedicated infrastructure for Materiovigilance activities.
The On-premise delivery mode gained favor among healthcare professionals and organizations due to its localized accessibility and control. Many preferred this method as it allowed them to manage Materiovigilance processes within their own physical environment, ensuring a sense of security and adherence to regulatory standards.
On the other hand, Cloud-Based delivery mode, while trailing slightly behind, displayed substantial growth, accounting for the remaining market share. This mode’s allure lies in its flexibility and scalability, offering users the advantage of remote access, real-time collaboration, and cost-efficiency. Healthcare entities seeking agility and seamless integration found Cloud-Based solutions appealing, driving the steady rise in its market presence.
As the Materiovigilance market continues to evolve, both On-premise and Cloud-Based delivery modes are expected to witness further developments. The On-premise segment may maintain its stronghold, especially among those emphasizing data control, while Cloud-Based solutions are poised to expand their influence by catering to the dynamic needs of healthcare professionals in an increasingly interconnected and digital healthcare landscape.
Application Analysis
In 2023, the Materiovigilance market witnessed a notable trend with the Diagnostic Application segment emerging as a frontrunner, securing a robust market position by commanding over 32.6% of the total share. This segment’s dominance can be attributed to its pivotal role in ensuring the safety and efficacy of diagnostic materials and devices.
The Therapeutic Application segment, comprising a diverse range of medical interventions, also played a significant role, capturing considerable attention and contributing substantially to the overall market landscape. Its share reflected a solid presence, providing assurance in the vigilance of materials used in various therapeutic applications.
Furthermore, the Surgical Application segment exhibited a noteworthy presence in the Materiovigilance market, emphasizing the critical importance of monitoring materials employed in surgical procedures. This segment, accounting for a substantial market share, underscored the growing emphasis on safety protocols and material vigilance within surgical settings.
Research Application, with its focus on advancements and innovations in medical materials, showcased a promising trajectory within the Materiovigilance market. This segment’s contribution was characterized by ongoing efforts to enhance the safety and performance of materials utilized in research settings.
Beyond these primary segments, the Materiovigilance market also benefited from the contributions of other applications. These miscellaneous applications, though diverse, collectively played a vital role in shaping the market landscape.
End Users Analysis
In 2023, the Materiovigilance market witnessed a notable landscape, with the Original Equipment Manufacturers (OEM) segment securing a predominant market position by capturing more than a 46.8% share. This segment played a pivotal role in shaping the Materiovigilance industry, reflecting its vital role in ensuring the safety and efficacy of medical devices and materials.
Contract Research Organizations (CROs) emerged as another significant player in the Materiovigilance market, contributing to advancements in research and development. With their expertise and streamlined processes, CROs facilitated efficient monitoring of materials’ safety, enhancing the overall Materiovigilance framework.
The Business Process Outsourcing (BPO) segment made noteworthy strides in 2023, offering valuable outsourcing solutions to streamline Materiovigilance activities. BPOs became integral partners for organizations seeking to optimize operational efficiency and compliance in managing materials’ safety information.
Furthermore, the market dynamics were influenced by various players falling under the “Others” category. These diverse participants added a layer of complexity and innovation to the Materiovigilance landscape, contributing unique perspectives and solutions to address safety concerns related to materials used in the healthcare industry.
Key Market Segments
Delivery Mode
- On-premise
- Cloud-Based
Application
- Diagnostic Application
- Therapeutic Application
- Surgical Application
- Research Application
- Others
End Users
- Contract Research Organization
- Business Process Outsourcing
- Original Equipment Manufacturers
- Others
Drivers
Stringent Regulatory Standards
The Materiovigilance market is propelled by an increasing focus on adherence to stringent regulatory standards. Governments worldwide are tightening regulations to ensure the safety and efficacy of medical devices, driving the demand for robust materiovigilance systems that can monitor and report adverse events effectively.
Rising Incidence of Adverse Events
The escalating incidence of adverse events associated with medical devices is a significant driver. As the use of complex devices grows, there is a heightened need for materiovigilance solutions that can swiftly identify and address potential risks, ensuring patient safety and regulatory compliance.
Advancements in Technology
Continuous advancements in technology, such as artificial intelligence and data analytics, are playing a pivotal role in the Materiovigilance market. These technologies enable more efficient data processing, analysis, and identification of patterns, enhancing the overall performance of materiovigilance systems.
Globalization of Medical Device Industry
The increasing globalization of the medical device industry is fostering market growth. With companies expanding their reach across borders, there is a growing demand for materiovigilance solutions that can harmonize and streamline adverse event reporting on a global scale, ensuring a unified and compliant approach.
Restraints
Complex Regulatory Landscape
The Materiovigilance market faces challenges due to the complexity of the global regulatory landscape. Varied regulatory requirements across regions pose obstacles for companies in terms of compliance, reporting, and maintaining consistency, hindering the seamless operation of materiovigilance systems.
Data Security Concerns
The rising concern over data security and privacy is a significant restraint. Materiovigilance systems deal with sensitive patient and product information, necessitating robust cybersecurity measures. The challenges associated with ensuring the confidentiality and integrity of data may impede the widespread adoption of materiovigilance solutions.
High Implementation Costs
The initial high costs associated with implementing materiovigilance systems act as a deterrent. Companies may hesitate to invest in these solutions due to budget constraints, particularly small and medium-sized enterprises, limiting the overall market penetration of materiovigilance technologies.
Lack of Standardization
The lack of standardized practices for materiovigilance reporting poses a challenge. Inconsistencies in reporting formats and criteria hinder effective communication and collaboration between stakeholders, making it essential to establish standardized protocols for adverse event reporting in the Materiovigilance market.
Opportunities
Increasing Adoption of Cloud-Based Solutions
The Materiovigilance market is poised for growth with the rising adoption of cloud-based solutions. Cloud platforms offer scalability, accessibility, and real-time data sharing, addressing the industry’s need for efficient and collaborative materiovigilance systems.
Emerging Markets and Untapped Regions
Untapped markets and emerging regions present significant growth opportunities. As healthcare infrastructure improves in developing regions, there is a growing demand for materiovigilance solutions, creating a potential market for companies willing to expand their presence in these areas.
Integration of Blockchain Technology
The integration of blockchain technology offers a promising growth avenue. Blockchain ensures data integrity, traceability, and security, addressing key concerns in materiovigilance. Its decentralized nature enhances transparency and trust in adverse event reporting, fostering the adoption of blockchain-based materiovigilance solutions.
Collaborations and Partnerships
Collaborations and partnerships within the healthcare ecosystem present strategic growth opportunities. Building synergies with regulatory bodies, healthcare providers, and technology partners can facilitate the development and adoption of comprehensive materiovigilance solutions, driving market growth through shared expertise and resources.
Trends
Real-Time Monitoring and Surveillance
The Materiovigilance market is witnessing a trend towards real-time monitoring and surveillance. The demand for instantaneous detection and response to adverse events is driving the integration of technologies that enable continuous and real-time data monitoring, enhancing the effectiveness of materiovigilance systems.
Focus on Patient-Centric Materiovigilance
There is a noticeable shift towards patient-centric materiovigilance practices. Recognizing the importance of patient perspectives in adverse event reporting, companies are incorporating patient feedback and engagement strategies into their materiovigilance systems, aligning with a more patient-centric approach.
AI-Powered Signal Detection
Artificial Intelligence (AI) is revolutionizing signal detection in materiovigilance. The use of AI algorithms and machine learning enhances the capability to identify subtle patterns and signals within large datasets, providing a more accurate and efficient means of detecting potential safety issues associated with medical devices.
Focus on Post-Market Surveillance
The Materiovigilance market is experiencing a trend towards intensified post-market surveillance. As regulatory scrutiny extends beyond pre-market approval, there is a growing emphasis on continuous monitoring of devices once they enter the market, ensuring ongoing safety and compliance through robust materiovigilance practices.
Regional Analysis
In 2023, North America emerged as a frontrunner in the Materiovigilance Market, establishing a dominant market position with a remarkable 46.2% share. The region’s robust standing is further underscored by its substantial market value, reaching USD 35.3 billion for the year.
North America boasts a highly developed healthcare infrastructure, marked by cutting-edge medical facilities, advanced research centers, and a strong regulatory framework. This foundation facilitates efficient materiovigilance practices and ensures swift response mechanisms.
The region adheres to stringent regulatory standards and compliance measures, ensuring a rigorous oversight of medical devices and materials. This commitment to regulatory excellence instills confidence among market stakeholders, fostering a secure environment for materiovigilance activities.
North America is at the forefront of technological innovation, with a constant influx of state-of-the-art medical technologies and devices. The integration of advanced materials and technologies in healthcare practices necessitates a vigilant approach to materiovigilance, further driving the demand for robust surveillance systems.
The rising awareness and reporting of adverse events associated with medical devices contribute to the region’s emphasis on materiovigilance. Heightened awareness among healthcare professionals and the general populace has led to a proactive approach in monitoring and addressing potential risks.
The collaborative efforts between regulatory bodies, healthcare providers, and industry stakeholders play a pivotal role in fostering materiovigilance in North America. Shared knowledge and coordinated initiatives contribute to the effective management of material-related risks.

Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the dynamic landscape of the Materiovigilance Market, several key players play pivotal roles in shaping its trajectory. AssurX stands out as a prominent player, leveraging its expertise to provide robust solutions and contribute significantly to market growth. The company’s commitment to quality and innovation positions it as a key influencer in the Materiovigilance space.
Sparta Systems, another major player, brings valuable contributions to the market through its innovative technologies and comprehensive approach. The company’s focus on compliance and risk management further solidifies its standing, making it a key player with a substantial impact on Materiovigilance practices.
Oracle Corporation, a global giant, extends its influence into the Materiovigilance Market, offering advanced solutions and leveraging its vast technological resources. The company’s integrated systems contribute to the market’s efficiency, addressing the evolving needs of Materiovigilance stakeholders.
Xybion Corporation adds a unique perspective to the Materiovigilance Market, specializing in providing solutions that align with regulatory requirements. Its tailored approach and commitment to customer satisfaction position it as a key player driving positive changes within the Materiovigilance landscape.
Among other key players, each contributes distinctively to the Materiovigilance Market, collectively forming a diverse and competitive ecosystem. These players often introduce innovations, foster collaboration, and enhance the overall market dynamics.
Market Key Players
- AssurX
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems
- MDI Consultants
- QVigilance
- Qserve
- ZEINCRO
Recent Developments
- In 2024: Oracle signed significant sales contracts, notably in training AI large language models within the Oracle Cloud. This included over 30 AI sales contracts totaling more than $12.5 billion. These developments are part of Oracle’s strategy to leverage AI demand to drive growth, exemplified by Oracle’s record-level sales that pushed their Remaining Performance Obligations (RPO) up by 44% to $98 billion.
- In November 2023: Xybion Corporation acquired Autoscribe Informatics, a U.K.-based provider of Laboratory Information Management System (LIMS) software. This strategic move enhances Xybion’s capabilities in lab digitization and information management, supporting industries such as life sciences, petrochemicals, and biobanking. This acquisition marks a significant expansion for Xybion in various global markets.
- In December 2020: Honeywell International Inc. acquired Sparta Systems from New Mountain Capital in an all-cash transaction valued at $1.3 billion. This acquisition is set to enhance Honeywell’s capabilities in the life sciences sector by integrating Sparta’s enterprise quality management software, including its next-generation SaaS platform, into Honeywell’s existing offerings. This move aims to improve quality management for life sciences companies, facilitating better compliance and faster time to market for new medical therapies.
Report Scope
Report Features Description Market Value (2023) USD 76.6 Bn Forecast Revenue (2033) USD 162.4 Bn CAGR (2024-2033) 7.8% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Delivery Mode (On-premise, Cloud-Based), By Application (Diagnostic Application, Therapeutic Application, Surgical Application, Research Application, Others), By End Users (Contract Research Organization, Business Process Outsourcing, Original Equipment Manufacturers, Others) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape AssurX, Sparta Systems, Oracle Corporation, Xybion Corporation, Sarjen Systems, MDI Consultants, QVigilance, Qserve, ZEINCRO, and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) 
-
-
- AssurX
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems
- MDI Consultants
- QVigilance
- Qserve
- ZEINCRO










