Global Huntingtons Disease Treatment Market Analysis By Treatment (Symptomatic Treatment, Disease-modifying Therapies), By End Use (Hospital Pharmacy, Retail Pharmacy, E-commerce), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: June 2025
- Report ID: 117818
- Number of Pages: 313
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
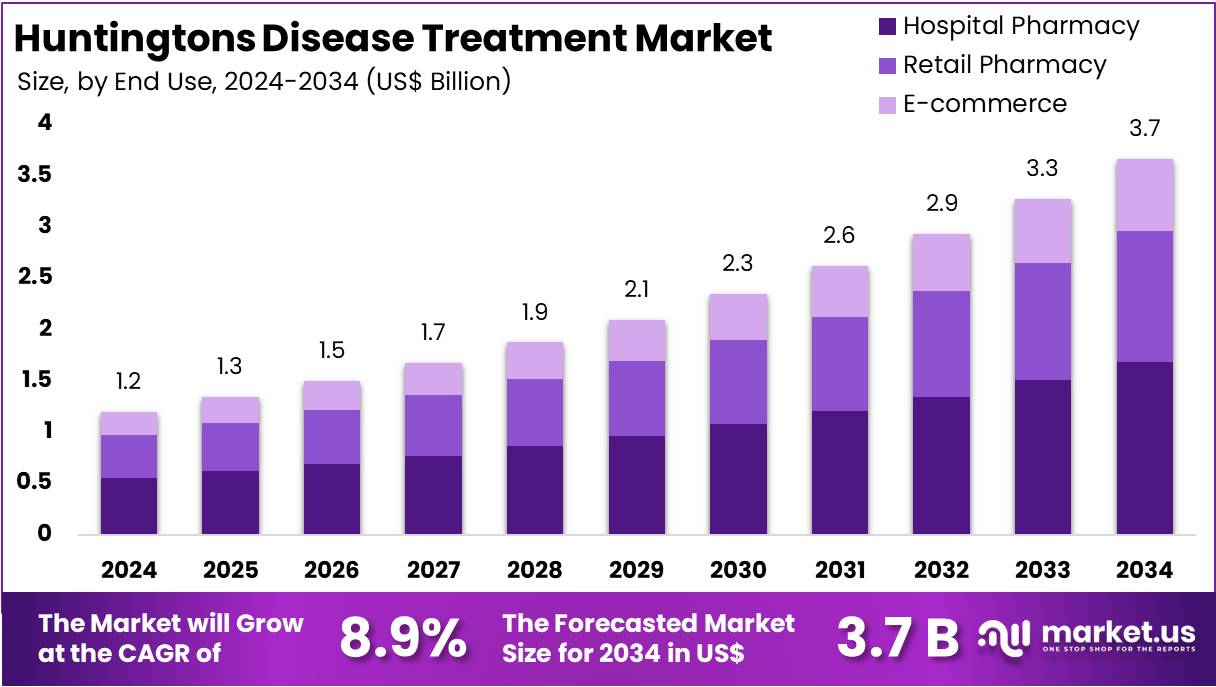
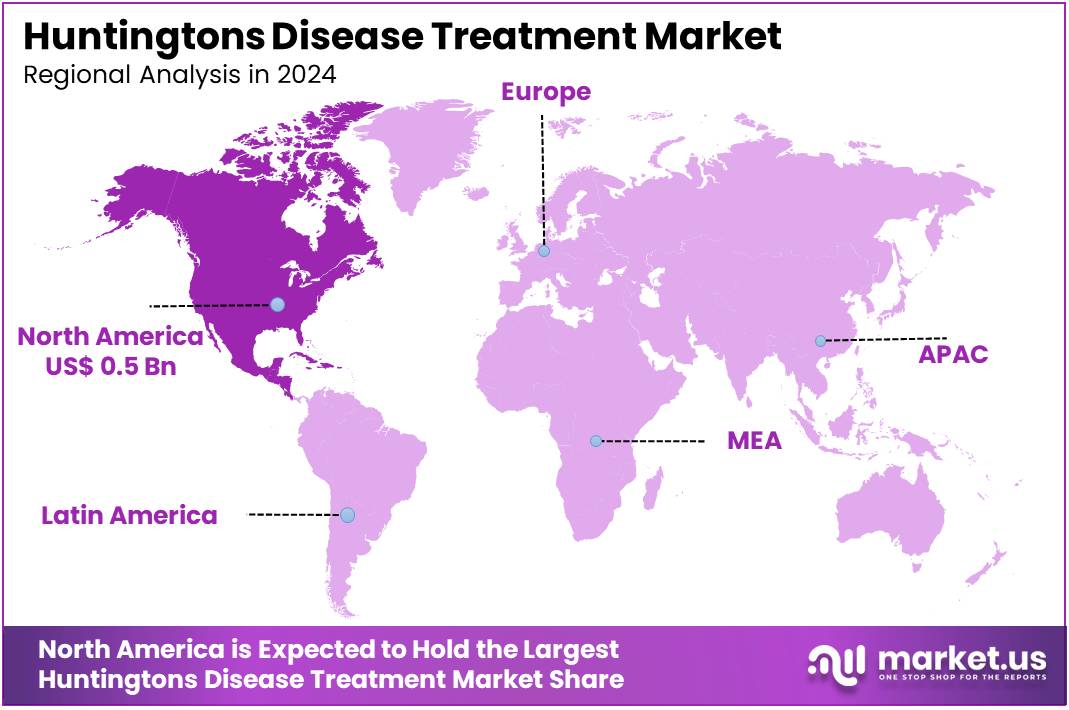
The Global Huntingtons Disease Treatment Market size is expected to be worth around US$ 3.7 Billion by 2034, from US$ 1.2 Billion in 2024, growing at a CAGR of 8.9% during the forecast period from 2025 to 2034. North America held a dominant market position, capturing more than a 41.7% share and holds US$ 0.5 Million market value for the year.
Huntingtons Disease is a rare, inherited neurodegenerative disorder that progressively impairs movement, cognition, and mental health. It is caused by a mutation in the HTT gene, leading to the gradual breakdown of nerve cells in the brain. According to MedlinePlus, a resource from the U.S. National Institutes of Health (NIH), Huntingtons Disease affects approximately 3 to 7 per 100,000 individuals of European ancestry. This chronic condition has no known cure, and treatment strategies focus on symptom management and improving patient quality of life.
Currently, available treatments aim to ease the motor and psychiatric symptoms associated with the disease. For instance, tetrabenazine is often prescribed to control involuntary movements (chorea), while antidepressants and antipsychotics help manage mood disorders. In addition to pharmacological therapies, supportive interventions such as physical, occupational, and speech therapy are widely used to enhance patient independence and communication capabilities. These combined approaches form the foundation of current clinical care for Huntington’s patients.
Market interest is steadily growing due to increasing knowledge about the molecular basis of the disease. This deeper understanding is driving the development of targeted and disease-modifying therapies. For example, several pharmaceutical and biotech companies are actively exploring the potential of gene therapy and RNA-targeting approaches. Such treatments aim to address the root cause of Huntington’s, rather than just alleviating its symptoms, offering hope for long-term disease control.
According to a study published in the Frontiers journal, the average duration from symptom onset to death in Huntingtons Disease is approximately 20 years. This long disease course highlights the urgent need for effective interventions that can slow progression. Current research pipelines include a range of investigational drugs in clinical trials, with the goal of reducing the production or aggregation of the mutant huntingtin protein responsible for neurodegeneration.
The Huntingtons Disease treatment market is in a phase of active innovation. While symptom-focused care remains the primary standard, advances in gene-based and disease-modifying therapies show strong potential. With rising clinical trial activity and expanding research funding, the future of Huntingtons Disease treatment is cautiously optimistic, aiming to change the course of this devastating illness.
Key Takeaways
- The global Huntington’s Disease Treatment Market is projected to reach USD 3.7 billion by 2034, rising from US$ 1.2 billion in 2024 at an 8.9% CAGR.
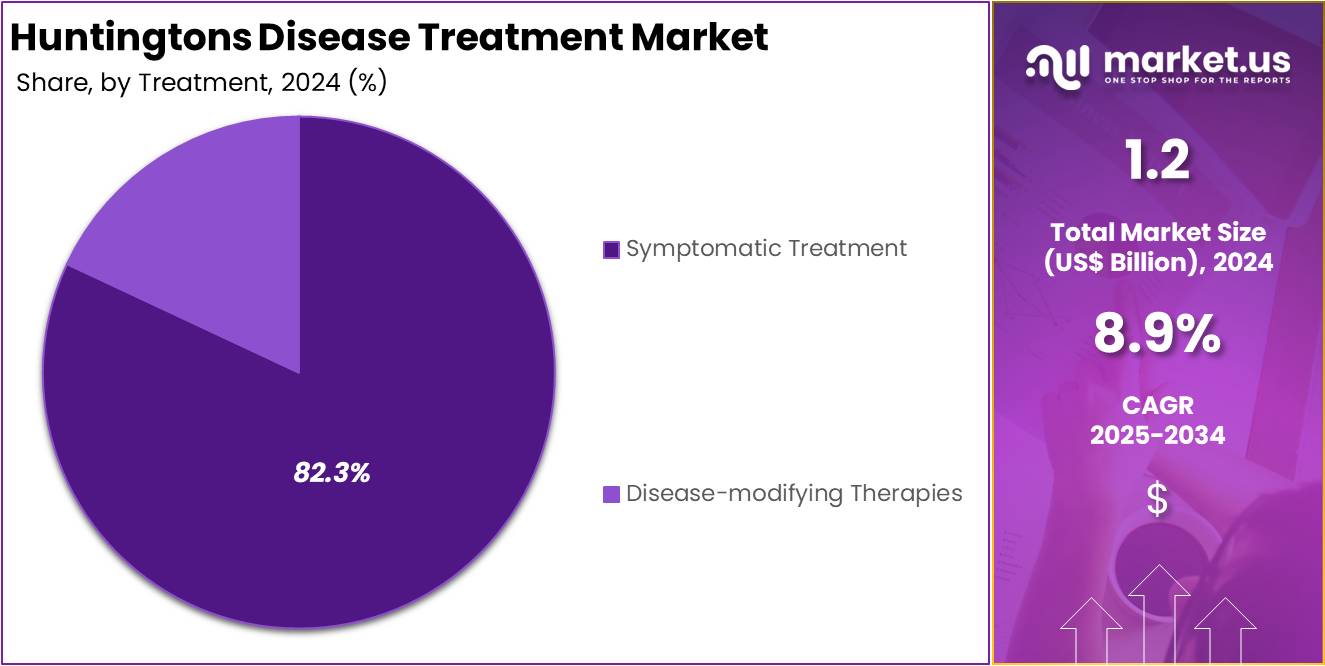
- In 2024, symptomatic treatments led the market by accounting for over 82.3% of the treatment segment, driven by their widespread use for managing disease symptoms.
- Hospital pharmacies dominated the end use segment in 2024, securing more than a 57.8% market share due to high patient preference for institutional care settings.
- North America held the leading regional market position in 2024, capturing a 41.7% share with an estimated value of US$ 0.5 billion that year.
Treatment Analysis
In 2024, the Symptomatic Treatment section held a dominant market position in the Treatment segment of Huntington’s Disease Treatment Market, and captured more than a 82.3% share. This dominance was driven by the lack of curative therapies and the widespread use of symptom management options. Patients typically receive medications such as tetrabenazine or deutetrabenazine to control involuntary movements. Antidepressants and antipsychotics are also commonly prescribed to manage mood and behavioral symptoms linked to Huntington’s Disease.
Experts observed that non-pharmacological support, such as speech, physical, and occupational therapy, played a vital role in improving daily functioning. These therapies are widely adopted in clinical settings to help patients retain motor and communication abilities for longer. Government health sources, including the National Institute of Neurological Disorders and Stroke (NINDS), have confirmed that current approaches mainly aim to reduce symptoms. As a result, the symptomatic treatment approach remains the primary option available to most healthcare providers and patients.
On the other hand, disease-modifying therapies are still under development. Many are in the clinical trial phase and are yet to receive regulatory approval. These include emerging methods like gene therapy and RNA-targeting treatments. While their future potential is promising, their limited clinical availability has kept their market share low. Due to proven outcomes and easier access, symptomatic treatments continue to dominate the overall Huntington’s Disease treatment landscape.
End Use Analysis
In 2024, the Hospital Pharmacy section held a dominant market position in the End Use Segment of Huntington’s Disease Treatment Market and captured more than a 57.8% share. This was mainly due to the need for in-hospital monitoring and professional management of complex symptoms. Hospital pharmacies are often responsible for dispensing drugs that treat movement disorders and psychiatric complications. These medications, such as tetrabenazine and antipsychotics, require strict supervision. Hospitals also provide tailored care for patients with advanced disease stages.
Retail pharmacies followed as the second-leading segment. They served a growing number of patients who manage their condition outside the hospital. These outlets offer prescription refills and over-the-counter support. Patients with milder symptoms often prefer visiting local pharmacies. The easy access and familiarity with retail setups also help boost demand. In many regions, increased awareness of neurological disorders has contributed to greater prescription volumes through retail pharmacy channels.
The e-commerce segment remained smaller but showed noticeable growth. This trend was supported by digital health expansion and patient interest in home delivery services. Online platforms allow access to specialty medicines without physical visits. This is especially useful for those living in remote areas or requiring long-term medication. However, challenges such as prescription verification and regulatory compliance persist. Despite these issues, the segment is expected to grow further as telehealth and e-pharmacy regulations evolve globally.
Key Market Segments
By Treatment
- Symptomatic Treatment
- Disease-modifying Therapies
By End Use
- Hospital Pharmacy
- Retail Pharmacy
- E-commerce
Drivers
Advancements in Personalized Medicine and Genomic Research
The emergence of personalized medicine has become a major driver in the Huntington’s disease treatment market. This approach focuses on tailoring therapies based on a patient’s genetic makeup, disease stage, and drug response. With the help of genomic technologies, healthcare providers can identify the most suitable treatment strategies. This not only improves clinical outcomes but also reduces the risk of side effects. Precision medicine enhances the ability to target Huntington’s disease more effectively, increasing treatment success rates in both early and advanced stages.
Genetic testing plays a central role in this shift toward personalized care. It allows for early diagnosis and assessment of risk in individuals with a family history of Huntington’s disease. Pharmacogenomic studies help determine how specific genetic mutations influence drug efficacy and tolerability. These insights are crucial in guiding physicians to select the most appropriate therapies. As a result, treatment becomes more efficient, and patients benefit from fewer adverse reactions and better overall management of the disease.
Alongside personalized care, growing research and development efforts are accelerating innovation in Huntington’s disease treatment. Pharmaceutical firms, research institutions, and public health agencies are investing in discovering new drug targets and therapeutic options. Clinical trials are underway for small molecules and other experimental treatments. Furthermore, stem cell therapy and neuroprotective agents are being investigated to slow disease progression. These R&D efforts represent a significant boost to long-term market growth.
Restraints
Challenges Due to Complex Disease Pathophysiology
One of the key restraints in the Huntington’s disease treatment market is the complex pathophysiology of the disorder. Huntington’s disease presents with a wide array of symptoms that affect motor function, cognitive abilities, and psychiatric health. Each symptom type may require a separate therapeutic approach, making treatment strategies more complicated. Furthermore, patient-to-patient variability in symptom presentation challenges the development of a one-size-fits-all treatment. This heterogeneity hampers the effectiveness of standardized therapies and adds layers of complexity to clinical management.
The disease is influenced by a mix of genetic, neurobiological, and environmental factors. These interwoven mechanisms create obstacles in identifying clear therapeutic targets. Efforts to address this multifactorial disease have been slowed by the difficulty in pinpointing the biological processes responsible for progression. As a result, many potential treatments fail to show consistent effectiveness across the patient population. This uncertainty discourages investment in drug development and makes regulatory approval more difficult due to inconsistent clinical outcomes.
Another significant limitation is the absence of reliable biomarkers for monitoring disease progression and treatment efficacy. Without validated biomarkers, clinical trial designs become more complex and time-consuming. Measuring outcomes accurately is difficult, increasing the risk of failed trials. This lack of standard measurement tools also restricts post-market assessment and personalized treatment adjustments. Together, these issues contribute to slower innovation and market growth in Huntington’s disease therapy.
Opportunities
Advancements in Genetic Therapies and Personalized Treatment Approaches
The Huntington’s disease treatment market is witnessing a major opportunity due to advances in genetic research. Emerging gene-editing tools such as CRISPR-Cas9 show promise in addressing the root cause of the disease. These technologies aim to correct or silence the mutated HTT gene, offering hope for long-term treatment. Several ongoing preclinical and early-stage trials are investigating their efficacy. If proven successful, such gene therapies may shift the market from symptom control to actual disease modification, representing a transformative shift in treatment strategies.
Another key opportunity lies in the development of disease-modifying therapies. Unlike current options that manage symptoms, these therapies aim to delay or stop disease progression. Clinical trials are underway for drugs that target neurodegenerative processes, protein aggregation, and mitochondrial dysfunction. These investigational drugs have shown encouraging results in early stages. Their potential approval may not only expand treatment choices but also improve the quality of life for patients, making them a critical driver of market growth in the near future.
Collaborations across academic institutions, biotech firms, and patient advocacy groups are accelerating innovation. The growing use of precision medicine is allowing treatments to be tailored to an individual’s genetic profile. This shift toward personalized care enhances therapeutic effectiveness. Such advancements reflect a proactive research environment that supports innovation. As a result, the market is expected to see a surge in investment and new product development in the coming years.
Trends
Advancing Toward Gene-Centric and Personalized Huntington’s Disease Therapies
The treatment landscape for Huntington’s disease is undergoing a major transformation. Precision medicine is emerging as a key approach, with genetic testing and biomarkers enabling therapies tailored to individual patient profiles. This shift supports the development of personalized treatment strategies. These innovations are helping clinicians manage the disease more effectively by targeting its root causes. As understanding of the disease’s genetic nature deepens, precision tools are likely to become central in clinical decision-making and treatment optimization.
Gene-editing technologies, particularly CRISPR-Cas9, are drawing significant attention in research circles. These tools offer the possibility of directly altering the genetic mutation that causes Huntington’s disease. Efforts in gene therapy are accelerating, with a focus on modifying or correcting the HTT gene. Early-stage trials of RNA-targeted and gene-silencing therapies are also showing promise. These techniques aim to reduce the toxic protein levels associated with the condition. Their development marks a shift from symptom management to potential disease modification.
Collaboration is becoming a cornerstone of progress in this field. Multidisciplinary partnerships between academic institutions, biotech firms, and pharmaceutical companies are increasing. These alliances are helping to fast-track the drug development process. Shared research platforms and clinical data are enabling faster innovation cycles. Together, these collaborations and gene-based solutions are defining a new era in Huntington’s disease treatment, offering hope for more effective and lasting outcomes.
Impact of Macroeconomic / Geopolitical Factors
Inflation significantly impacts the Huntington’s disease treatment market by increasing overall healthcare costs. Rising expenses in research, clinical trials, drug manufacturing, and hospital care directly affect pricing strategies. As a result, affordability becomes a concern for both providers and patients. Pharmaceutical companies may be forced to adjust development timelines or limit investments in niche therapies. Higher operational costs also challenge small biotechnology firms. This financial pressure influences market accessibility, pricing models, and the long-term sustainability of innovation in Huntington’s disease treatment.
Currency exchange rates affect global partnerships, investment flows, and the cross-border movement of pharmaceutical goods and technologies. Volatile exchange rates can raise the cost of imported raw materials, active pharmaceutical ingredients (APIs), and specialized equipment used in Huntington’s disease drug development. For multinational firms, fluctuations in local currencies can lead to losses in revenue or profit margins. These risks influence strategic decisions related to international licensing, clinical trials in foreign markets, and the regional supply chain stability for Huntington’s disease therapeutics.
Government policies remain critical in shaping the market environment for Huntington’s disease treatments. Supportive measures like orphan drug incentives, fast-track approvals, and R&D grants encourage pharmaceutical innovation. Regulatory bodies that ease approval timelines enable quicker access to life-saving therapies. However, pricing controls, patent restrictions, and limited reimbursement frameworks can discourage new entrants and reduce profitability. As a result, investor confidence may decline. Effective public policy is essential to promote equitable treatment access while encouraging innovation in this complex and high-need therapeutic area.
Regional Analysis
North America is leading the Huntingtons Disease Treatment Market
In North America, particularly in the United States, the Huntington’s disease (HD) treatment market is supported by a strong healthcare infrastructure and dedicated research institutions. The region accounted for the largest market share in 2024, capturing approximately 41.7%. This dominance is attributed to increased awareness, early diagnosis, and focused investment in rare diseases. The presence of key pharmaceutical and biotech companies has enhanced research output. These firms actively engage in clinical trials and drug development initiatives, fostering growth and innovation in the HD treatment landscape.
Regulatory support from the U.S. Food and Drug Administration (FDA) contributes to the approval and monitoring of HD therapies. This has created a favorable environment for research and commercialization. Patient advocacy groups play a key role by promoting awareness, offering support services, and influencing healthcare policies. Their initiatives have improved funding, access to care, and treatment options for HD patients. The combined efforts of regulators, industry players, and advocacy groups continue to drive market progress in North America.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
In the Asia Pacific region, the Huntington’s disease (HD) treatment market is expanding. However, it faces several challenges. These include limited healthcare infrastructure in some countries, inconsistent regulatory frameworks, and restricted access to new therapies. Nations like Japan, South Korea, and Australia have advanced health systems and research efforts in HD. Yet, many areas in the region still struggle with access and awareness. These disparities affect patient care and delay the adoption of emerging treatment options in less developed healthcare settings.
To address these barriers, strong collaboration is essential. Partnerships with global pharmaceutical firms, research organizations, and advocacy groups help drive progress. Awareness campaigns and educational efforts also play a key role. These initiatives improve diagnosis, care quality, and support for patients and caregivers. Additionally, harmonizing regulatory policies and investing in research infrastructure can help bridge existing gaps. Such efforts are critical to ensure broader, more equitable access to innovative Huntington’s disease treatments across Asia Pacific.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The Huntington’s disease treatment market remains highly competitive, shaped by innovation, strategic partnerships, and a robust focus on research. Key players collaborate with research organizations and academic centers to enhance drug development. These collaborations support clinical advancements and speed up market entry. Companies are diversifying their portfolios to include both symptomatic and disease-modifying therapies. This strategy helps address the multifaceted nature of Huntington’s disease. Such efforts are crucial for capturing broader patient populations and maintaining a strong position in an evolving therapeutic landscape.
H. Lundbeck A/S holds a significant market share due to its CNS specialization and its well-known product, Xenazine (tetrabenazine), used for chorea in Huntington’s disease. The company has secured broad regulatory approvals in North America and Europe. Continued investment in neurology-focused R&D reinforces its market leadership. Lundbeck’s extensive commercial infrastructure supports its strong international presence. These combined efforts enhance the company’s competitive advantage and enable it to respond to growing therapeutic demands across different regions.
Teva Pharmaceutical Industries Ltd. plays a leading role in the Huntington’s disease treatment market with its product Austedo (deutetrabenazine), approved by the U.S. FDA. The company offers a mix of branded and generic therapies. It has established a strong foothold in North America and Europe. Teva continues to focus on neurological innovation and product life cycle management. These strategic initiatives help the company maintain its relevance in a competitive field and ensure continued growth in the neurology space.
Bausch Health and Hetero are also active participants in the market. Bausch uses its retail and hospital distribution network to expand access to tetrabenazine-based treatments. The company focuses on optimizing its portfolio and geographic reach. Hetero, on the other hand, emphasizes affordable generic CNS drugs for emerging markets. It plays a key role in improving drug accessibility. Additionally, companies like Neurocrine Biosciences, uniQure, and Wave Life Sciences are developing advanced therapies such as gene editing and RNA-targeted treatments, signaling a shift toward precision medicine.
Huntingtons Disease Treatment Market Key Players Are
- H. Lundbeck A/S
- Teva Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.
- Hetero
- Lupin
- Hikma Pharmaceuticals PLC
- Dr. Reddy’s Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.
Recent Developments
- In April 2024: American Academy of Neurology (AAN) Annual Meeting, Teva Pharmaceuticals shared final outcomes from the Phase 4 START study, which assessed the real-world use of AUSTEDO® (deutetrabenazine) for treating chorea associated with Huntington’s disease. The study highlighted that approximately 76% of patients were able to complete the four-week titration process successfully. Treatment adherence exceeded 90%, and patients demonstrated a 33% average reduction in total maximal chorea (TMC) scores from baseline. Both patient and clinician feedback indicated high satisfaction, reinforcing AUSTEDO®’s effectiveness and its positive impact on treatment outcomes.
- August 2023: The U.S. Food and Drug Administration (FDA) approved Valbenazine, commercially known as INGREZZA, for the treatment of symptoms associated with Huntington’s disease. This therapy was developed by Neurocrine Biosciences.
- October 2023: The U.S. FDA granted orphan drug designation to SAGE-718, a therapeutic candidate developed by Sage Therapeutics, for the treatment of Huntington’s disease.
Report Scope
Report Features Description Market Value (2024) US$ 1.2 billion Forecast Revenue (2034) US$ 3.7 billion CAGR (2025-2034) 8.9% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Treatment (Symptomatic Treatment, Disease-modifying Therapies), By End Use (Hospital Pharmacy, Retail Pharmacy, E-commerce) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape H. Lundbeck A/S, Teva Pharmaceutical Industries Ltd., Bausch Health Companies Inc., Hetero, Lupin, Hikma Pharmaceuticals PLC, Dr. Reddy’s Laboratories Ltd., Sun Pharmaceutical Industries Ltd. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Huntingtons Disease Treatment MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample
Huntingtons Disease Treatment MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- H. Lundbeck A/S
- Teva Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.
- Hetero
- Lupin
- Hikma Pharmaceuticals PLC
- Dr. Reddy’s Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.










