Global Meningococcal Vaccines Market By Product Type (Multivalent Vaccines, Monovalent Vaccines, and Conjugate Vaccines), By Age Group (Children, Infants, Adolescents, and Adults), By Administration Route (Subcutaneous, Intramuscular, and Oral), By Distribution Channel (Hospitals, Clinics, and Pharmacies), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2034
- Published date: Feb 2025
- Report ID: 139467
- Number of Pages: 242
- Format:
-
keyboard_arrow_up
Quick Navigation
- Report Overview
- Key Takeaways
- Product Type Analysis
- Age Group Analysis
- Administration Route Analysis
- Distribution Channel Analysis
- Key Market Segments
- Drivers
- Restraints
- Opportunities
- Impact of Macroeconomic / Geopolitical Factors
- Latest Trends
- Regional Analysis
- Key Players Analysis
- Recent Developments
- Report Scope
Report Overview
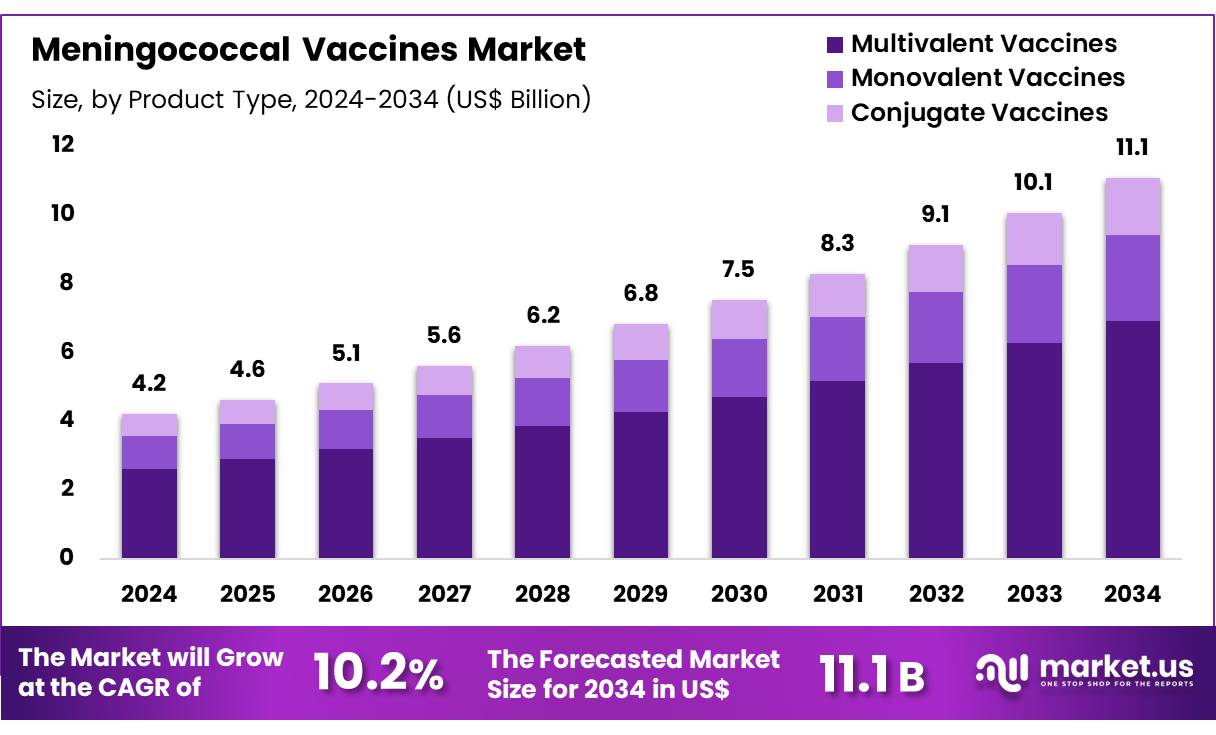
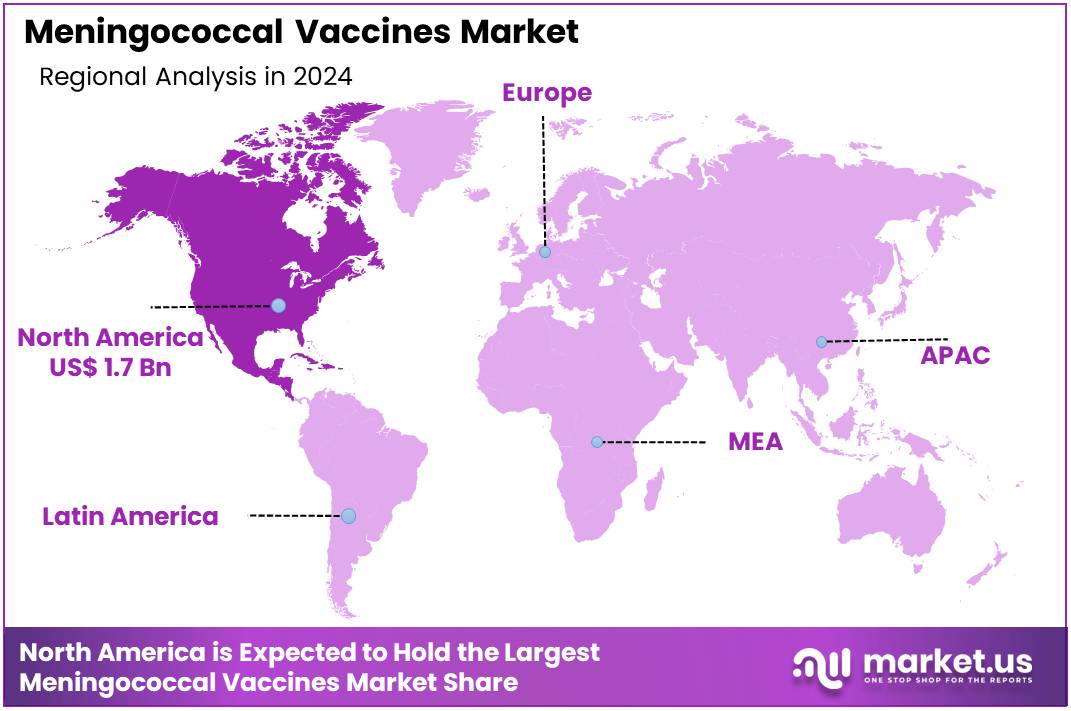
Global Meningococcal Vaccines Market size is expected to be worth around US$ 11.1 billion by 2034 from US$ 4.2 billion in 2024, growing at a CAGR of 10.2% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 40.1% share with a revenue of US$ 1.7 Billion.
Increasing awareness of the high mortality and morbidity associated with meningococcal diseases is driving the growth of the meningococcal vaccines market. These vaccines play a critical role in preventing meningitis and septicemia caused by Neisseria meningitidis, particularly in high-risk populations such as infants, adolescents, and military personnel. The rising prevalence of meningococcal disease outbreaks, as highlighted by WHO data showing significant rates in sub-Saharan Africa, underscores the need for widespread vaccination programs.
The “Meningitis Belt,” which stretches from Senegal to Ethiopia, remains a focal point, with over 800,000 cases and more than 7,500 deaths reported during the 2020-2021 meningitis season. In March 2023, GSK plc reported positive results from the Phase 3 clinical trial of its MenABCWY combo vaccine candidate, designed to protect against multiple meningococcal strains. The vaccine is set to be administered in two doses to individuals aged 10-25 years, addressing a broad demographic.
Recent trends show a shift towards combination vaccines, which offer protection against several pathogens with a single dose, improving vaccine coverage and compliance. Additionally, increasing government initiatives and the growing focus on universal immunization programs present significant opportunities for market expansion. As the global burden of meningococcal disease continues to demand innovative solutions, the market is poised for growth driven by new vaccine developments and improved distribution networks.
Key Takeaways
- In 2024, the market for Meningococcal Vaccines generated a revenue of US$ 4.2 billion, with a CAGR of 10.2%, and is expected to reach US$ 11.1 billion by the year 2034.
- The product type segment is divided into multivalent vaccines, monovalent vaccines, and conjugate vaccines, with multivalent vaccines taking the lead in 2024 with a market share of 62.4%.
- Considering age group, the market is divided into children, infants, adolescents, and adults. Among these, children held a significant share of 58.6%.
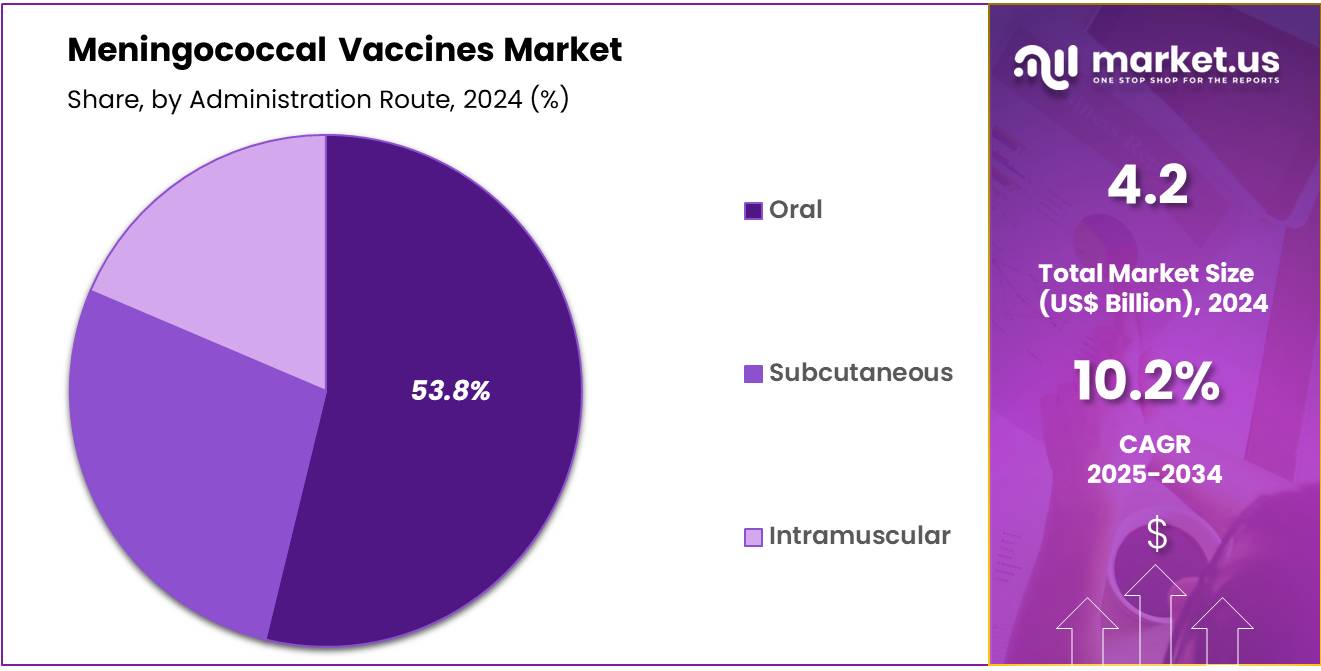
- Furthermore, concerning the administration route segment, the market is segregated into subcutaneous, intramuscular, and oral. The oral sector stands out as the dominant player, holding the largest revenue share of 53.8% in the Meningococcal Vaccines market.
- The distribution channel segment is segregated into hospitals, clinics, and pharmacies, with the hospitals segment leading the market, holding a revenue share of 56.9%.
- North America led the market by securing a market share of 40.1% in 2024.
Product Type Analysis
The multivalent vaccines segment led in 2024, claiming a market share of 62.4% owing to their ability to protect against multiple strains of Neisseria meningitidis in a single dose. The increasing prevalence of meningococcal infections, combined with the need for broad protection, is likely to drive the demand for multivalent vaccines. These vaccines are anticipated to be preferred in mass immunization campaigns, as they provide wider coverage and help control outbreaks more effectively.
The growing awareness of the importance of vaccination, coupled with advancements in vaccine formulations, is expected to contribute to the market expansion. Additionally, multivalent vaccines’ ability to address multiple serogroups in one injection is projected to improve compliance and vaccination rates, further fueling the growth of this segment.
Age Group Analysis
The children held a significant share of 58.6% due to the increasing focus on childhood immunization programs. Vaccinating children against meningococcal diseases has become a priority for health authorities due to the vulnerability of young children to these infections. The rising number of recommended vaccination schedules for children, particularly in the early years of life, is likely to contribute to the growth of this segment.
Governments and health organizations are expected to continue their efforts to expand vaccination coverage, especially in developing regions, where the incidence of meningococcal diseases remains high. The growing emphasis on preventive healthcare and the availability of vaccines that protect against multiple strains of meningococcus are anticipated to further accelerate the growth of this segment.
Administration Route Analysis
The oral segment had a tremendous growth rate, with a revenue share of 53.8% owing to the growing preference for non-invasive vaccination methods. Oral vaccines are expected to become more popular due to their ease of administration, particularly in mass vaccination campaigns or in regions with limited healthcare infrastructure. The convenience of oral vaccines, which eliminate the need for injections and reduce the risk of needle-related issues, is projected to improve patient acceptance and increase vaccination rates.
Additionally, advancements in oral vaccine formulations are expected to enhance their efficacy, making them more attractive for meningococcal immunization programs. As research into oral vaccine delivery systems progresses, the oral segment is likely to witness significant growth, particularly in areas where access to healthcare facilities is limited.
Distribution Channel Analysis
The hospitals segment grew at a substantial rate, generating a revenue portion of 56.9% as hospitals continue to be a primary setting for administering vaccines, particularly for patients at high risk of meningococcal infections. Hospitals are anticipated to remain key distribution points for meningococcal vaccines due to their infrastructure, medical staff, and ability to manage emergency situations such as meningococcal outbreaks.
The rising prevalence of meningitis and the growing recognition of the importance of immunization in preventing infectious diseases are likely to drive the demand for vaccines within hospitals. Furthermore, as hospitals increasingly focus on offering comprehensive immunization services, the role of hospitals in vaccine distribution is expected to expand. Hospitals are projected to continue being central hubs for meningococcal vaccination programs, particularly for vulnerable populations, contributing to the growth of this segment.
Key Market Segments
By Product Type
- Multivalent Vaccines
- Monovalent Vaccines
- Conjugate Vaccines
By Age Group
- Children
- Infants
- Adolescents
- Adults
By Administration Route
- Subcutaneous
- Intramuscular
- Oral
By Distribution Channel
- Hospitals
- Clinics
- Pharmacies
Drivers
Growing Prevalence of Bacterial Meningitis Driving the Meningococcal Vaccines Market
Growing prevalence of bacterial meningitis is anticipated to drive the meningococcal vaccines market significantly. The Centers for Disease Control and Prevention estimate that 1.2 million cases of bacterial meningitis occur globally each year, highlighting the urgent need for prevention measures. Meningitis outbreaks, particularly in regions with limited healthcare access, emphasize the importance of widespread vaccination campaigns.
Governments and global health organizations prioritize meningococcal vaccination programs to reduce the disease burden and prevent fatalities. Pharmaceutical companies continue to develop advanced vaccines targeting multiple strains of meningococcus to enhance protection. Expanding immunization initiatives in schools and colleges contribute to higher vaccination rates, especially among adolescents and young adults. Travelers to high-risk regions increasingly adopt meningococcal vaccines as a preventive measure, driving market demand. Innovations in vaccine delivery systems, such as single-dose formulations, improve accessibility and compliance.
Collaborative efforts among research institutions, vaccine manufacturers, and public health agencies strengthen distribution networks. Increasing investments in R&D support the development of next-generation vaccines with improved efficacy and broader coverage. Rising awareness of meningitis prevention among healthcare providers and the general population further bolsters market growth. These factors underscore the critical role of vaccination in combating bacterial meningitis worldwide.
Restraints
High Costs Are Restraining the Meningococcal Vaccines Market
High costs of meningococcal vaccines are restraining the market. Advanced vaccine formulations targeting multiple strains involve significant research and development expenses. Manufacturing processes require stringent quality control measures, further increasing production costs. In low-income countries, where meningitis incidence is often higher, affordability remains a significant challenge. Limited funding for immunization programs restricts access to vaccines in these regions.
The cold chain infrastructure required for vaccine storage and transportation adds to operational expenses, particularly in rural areas. Insurance coverage gaps for meningococcal vaccination in several countries limit adoption rates. Addressing these cost-related barriers requires innovative solutions, including subsidies, public-private partnerships, and cost-efficient production methods to ensure broader accessibility.
Opportunities
Rising Awareness as an Opportunity for the Meningococcal Vaccines Market
Rising awareness about meningococcal disease prevention presents a significant opportunity for the meningococcal vaccines market. In August 2021, DoSomething.Org and the National Foundation for Infectious Diseases launched the “Complete What’s Missing Program,” focusing on educating young people about meningitis prevention. GSK plc’s ASK2BSure campaign, also launched in August 2021, encouraged parents to discuss Meningitis B vaccination with healthcare providers.
These initiatives highlight the growing emphasis on preventive healthcare and immunization awareness. Public health campaigns targeting schools, colleges, and community centers enhance vaccination uptake among at-risk groups. Social media and digital platforms amplify outreach efforts, ensuring widespread dissemination of educational content. Collaborations between non-profits, healthcare providers, and vaccine manufacturers strengthen awareness initiatives.
Increased public understanding of meningitis risks drives proactive vaccination decisions. Government-supported awareness campaigns, combined with expanded immunization coverage, boost market growth. These trends position awareness programs as a critical driver in enhancing meningococcal vaccine adoption and reducing the global burden of bacterial meningitis.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors have a significant impact on the meningococcal vaccines market. On the positive side, increasing healthcare investments, particularly in emerging markets, boost vaccine accessibility and distribution. Rising awareness of vaccine-preventable diseases and government support for immunization programs drive demand for meningococcal vaccines.
However, economic downturns or budget cuts in public health spending may reduce the availability of vaccines, especially in low-income regions. Geopolitical issues, such as trade restrictions, political instability, or regulatory barriers, could disrupt the supply chain for vaccine production and distribution, impacting availability and pricing.
Additionally, varying healthcare policies and reimbursement practices across countries can create challenges in ensuring wide vaccine access. Despite these hurdles, growing government and international organization support for vaccination programs, particularly in high-risk areas, ensures a positive outlook for the market.
Latest Trends
Increased Funding for Child Immunization Driving the Meningococcal Vaccines Market:
Rising funding for child immunization is a key driver of growth in the meningococcal vaccines market. High investment in immunization programs is expected to increase vaccine coverage and reduce the incidence of meningococcal disease among children. The growing focus on childhood vaccinations is likely to improve public health outcomes and promote broader adoption of meningococcal vaccines worldwide.
Beginning in March 2023, Bexsero will be funded for the immunization of children up to 12 months old as part of the childhood immunization program, and for individuals aged 13 to 25 years. This initiative is anticipated to expand vaccine access, especially in regions with high disease prevalence, further fueling market growth. As funding continues to increase, more children will be protected, driving positive growth trends in the meningococcal vaccines market.
Regional Analysis
North America is leading the Meningococcal Vaccines Market
North America dominated the market with the highest revenue share of 40.1% owing to rising awareness of the importance of vaccination, the increasing prevalence of meningococcal disease, and strong public health initiatives. The region has experienced an uptick in the incidence of serious meningococcal infections, which has heightened the demand for effective vaccination programs. In March 2024, the Centers for Disease Control and Prevention (CDC) issued a warning about the increasing cases of serious meningococcal disease in the U.S., primarily caused by the Neisseria meningitidis serogroup Y.
This led to a renewed focus on immunization efforts, particularly in high-risk populations such as adolescents and college students, who are more susceptible to contracting the disease. The implementation of vaccination policies and campaigns, supported by healthcare providers and public health organizations, has contributed to the growth of the meningococcal vaccines market. Additionally, innovations in vaccine formulations and improved accessibility have further expanded the adoption of meningococcal vaccines, positioning the market for continued growth in North America.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to grow with the fastest CAGR owing to rising healthcare awareness, increasing incidences of meningococcal diseases, and improvements in vaccination infrastructure. As the region continues to develop its healthcare systems and adopt advanced vaccination strategies, countries such as China, India, and Japan are anticipated to see increased demand for meningococcal vaccines.
The growing prevalence of meningococcal infections, particularly in densely populated areas and among vulnerable populations such as children and young adults, is likely to spur the need for effective vaccination programs. Additionally, the increasing focus on preventive healthcare and the implementation of nationwide immunization campaigns are projected to drive market growth.
Government initiatives to improve vaccine accessibility and affordability, combined with rising investments in public health, are expected to further propel the meningococcal vaccines market in Asia Pacific. The region’s expanding healthcare infrastructure, coupled with the global push for universal vaccination, will contribute to the sustained growth of the meningococcal vaccines market.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the meningococcal vaccines market focus on strategies like developing multivalent vaccines to provide broader protection against multiple serogroups. Companies invest in R&D to innovate effective and long-lasting immunization options for diverse age groups.
Partnerships with governments and global health organizations help expand vaccination programs in regions with high disease prevalence. Geographic expansion into emerging markets with growing healthcare awareness supports broader access and adoption. Many players also emphasize affordability and compliance with regulatory standards to enhance public health outcomes.
Pfizer Inc. is a leading company in this market, offering vaccines like Trumenba, designed to protect against serogroup B meningococcal disease. The company focuses on innovation, strong distribution networks, and partnerships with health organizations to advance immunization efforts globally. Pfizer’s commitment to improving global health and its extensive vaccine portfolio solidify its position in the industry.
Top Key Players
- Valneva
- Serum Institute of India
- Sanofi
- Pfizer
- MassBiologics
- Hikma Pharmaceuticals
- GlaxoSmithKline
- Baxter International
- Astellas Pharma
Recent Developments
- In April 2024, GSK plc, a biopharma company, announced that the U.S. FDA had accepted its Biologics License Application (BLA) for the 5-in-1 meningococcal ABCWY vaccine candidate. This vaccine combines components from GSK’s Bexsero and Menveo vaccines, targeting the A, C, W, B, and Y meningococcal groups, which are responsible for the majority of invasive meningococcal disease cases globally. The FDA is expected to make a decision on the application by February 14, 2025.
- In October 2023, Pfizer, a leading global pharmaceutical and biotechnology company, announced that the U.S. FDA had approved PENBRAYA. This is the first pentavalent vaccine designed for adolescents and young adults (ages 10-25) to protect against meningococcal groups A, C, W, B, and Y. The vaccine combines components from Trumenba and Nimenrix, covering the most prevalent serogroups responsible for meningococcal disease worldwide.
Report Scope
Report Features Description Market Value (2024) US$ 4.2 billion Forecast Revenue (2034) US$ 11.1 billion CAGR (2025-2034) 10.2% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Multivalent Vaccines, Monovalent Vaccines, and Conjugate Vaccines), By Age Group (Children, Infants, Adolescents, and Adults), By Administration Route (Subcutaneous, Intramuscular, and Oral), By Distribution Channel (Hospitals, Clinics, and Pharmacies) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Valneva, Serum Institute of India, Sanofi, Pfizer, MassBiologics, Hikma Pharmaceuticals, GlaxoSmithKline, Baxter International, and Astellas Pharma. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Meningococcal Vaccines MarketPublished date: Feb 2025add_shopping_cartBuy Now get_appDownload Sample
Meningococcal Vaccines MarketPublished date: Feb 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Valneva
- Serum Institute of India
- Sanofi
- Pfizer
- MassBiologics
- Hikma Pharmaceuticals
- GlaxoSmithKline
- Baxter International
- Astellas Pharma









