Deferiprone Market Analysis By Type (Tablet, Oral Solution, Capsule), By Therapeutic Use (Iron Overload Disorders, Thalassemia Treatment, Sickle Cell Disease Treatment), By Indication (Transfusional Iron Overload, NTDT CAused Overload), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Apr 2025
- Report ID: 21025
- Number of Pages: 239
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
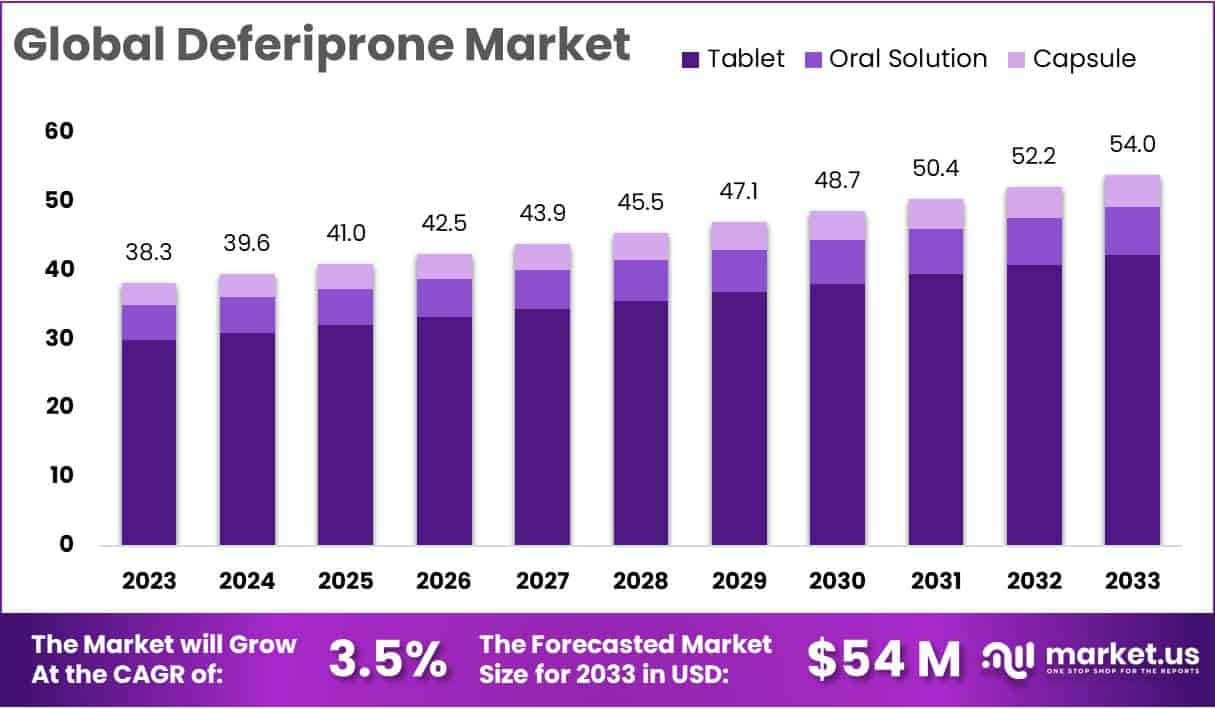
The Deferiprone Market Size is projected to reach approximately USD 54 million by 2033, up from USD 38.3 million in 2023. This represents a steady growth rate of 3.5% from 2024 to 2033.
Deferiprone (marketed under its brand name Ferriprox) is an FDA-approved iron chelator intended to address transfusional iron overload in patients suffering from thalassemia syndromes. Deferiprone is especially effective at eliminating toxic metal build-ups in order to treat severe thalassemia symptoms, providing much-needed relief. Used frequently in transfusion-dependent thalassemia cases, this drug counters iron accumulation caused by either disease progression or frequent blood transfusions.
Deferiprone has proven itself effective, yet comes with known adverse side effects including agranulocytosis, neutropenia, and gastrointestinal discomfort. Global sales for Deferiprone are projected to expand with growing prevalence of thalassemia as well as ongoing efforts to enhance accessibility – particularly among developing nations.
Deferiprone is well known as an effective means of treating chronic iron overload; however, its use raises many ethical considerations. Deferiprone is recognized for being potentially carcinogenic, genotoxic, and teratogenic which limits its application; typically used when existing chelation therapy fails due to reported side effects like nausea, vomiting, abdominal pain, joint pain zinc deficiency or hypersensitivity symptoms in some patients. Regardless of these challenges ongoing R&D activities exploring how Deferiprone can treat other rare disorders should drive global adoption contributing positively towards its market growth potential and global adoption as a whole.
The global Deferiprone market can be divided into three key sections, comprising formulation, indication and region. Formulations available include tablets, oral solutions and capsules; with tablets holding the greatest market share currently. Oral solutions are expected to experience greater growth. Among indications there are two subcategories; transfusional iron overload vs NTDT caused iron overload being most widely seen around the globe.
Hemochromatosis prevalence worldwide has steadily been on the rise, driving an expansion in deferiprone industry growth. According to American Diabetes Association estimates, hereditary hemochromatosis affects one of every 200 to 300 West individuals and stands as one of the most prevalent single gene disorders. Transfusion-dependent thalassemia cases will further drive deferiprone demand; according to World Health Organization reports from 2008 1.1% couples risk giving birth with hemoglobin disorders which affect 2.7% of births overall.
Deferiprone is likely to face challenges related to adverse reactions like chromaturia and nausea; nonetheless, growth should still be anticipated with increased funding from governments, private agencies, healthcare initiatives as well as healthcare initiatives – for instance in UAE where premarital screening screening was made mandatory in 2017. PLOS One Journal also published about this initiative back then which provided information regarding risk of having children with Thalassemia Major as reported in this campaign by their government.
Key Takeaways
- Market Growth: Deferiprone market is set to reach USD 54 million by 2033, growing steadily at 3.5% annually from 2024.
- Segment Dominance: In 2023, tablets held a substantial 78.6% market share, with oral solutions and capsules following closely.
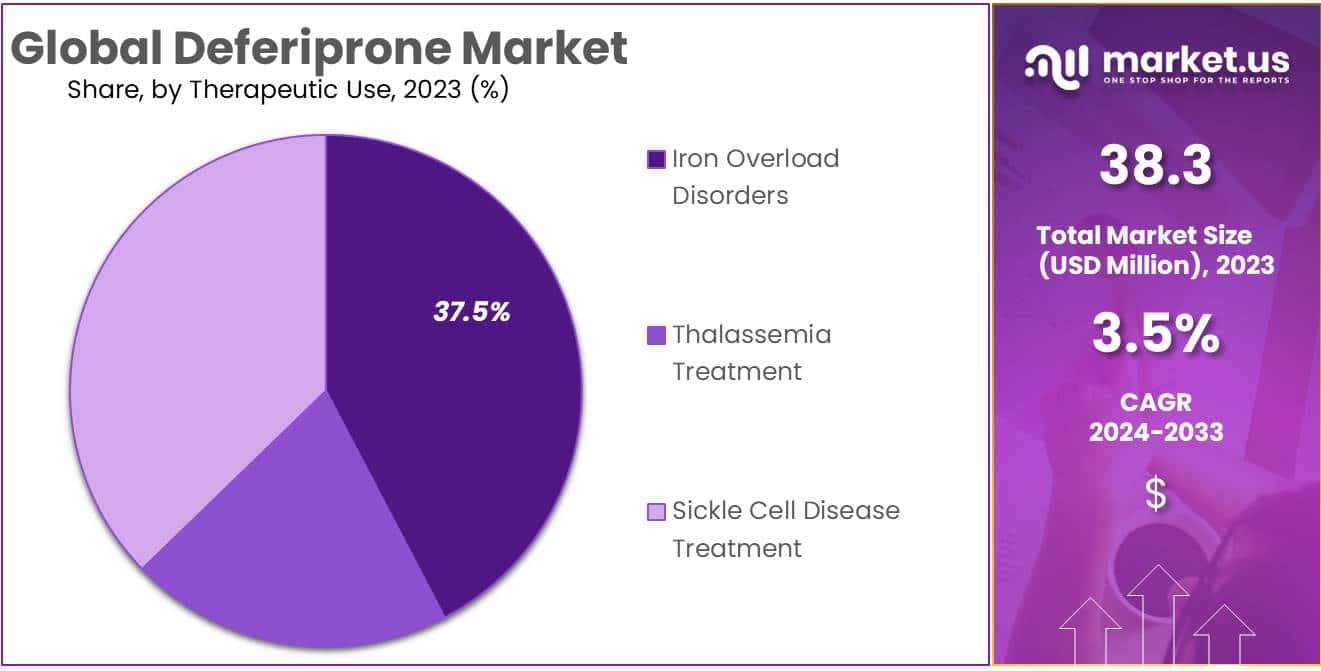
- Therapeutic Focus: Iron Overload Disorders commanded a leading 37.5% market share in 2023, driven by conditions like hemochromatosis.
- Indication Leadership: Transfusional Iron Overload dominated with a significant 61.1% market share in 2023.
- Key Drivers: Rising prevalence of iron disorders and ongoing R&D investments contribute to Deferiprone market growth.
- Challenges: Adverse reactions, patient compliance issues, and stringent regulatory approvals present obstacles to market expansion.
- Opportunities: Emerging markets offer growth potential, emphasizing strategic collaborations, personalized medicine, and pediatric applications.
- Noteworthy Trends: Increasing adoption of oral chelation therapies, patient-centric healthcare, and innovations in drug delivery systems are observed trends.
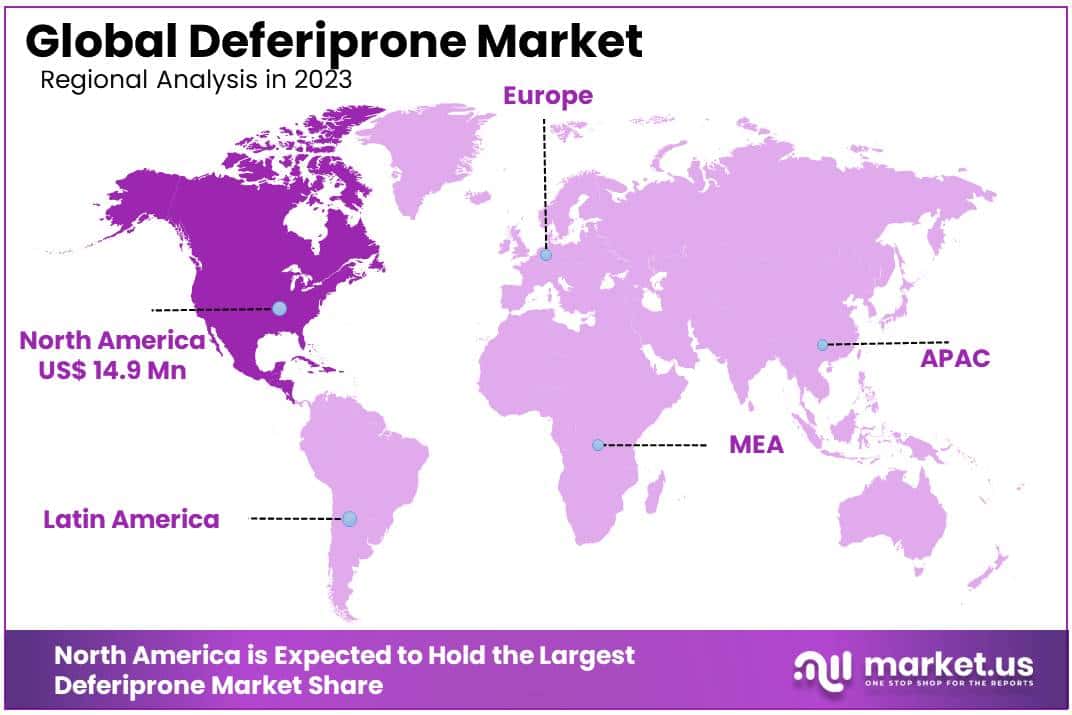
- Regional Dynamics: North America, with over 39.1% market share in 2023, leads due to a surge in iron disorders and robust healthcare infrastructure.
- Future Outlook: Anticipate market evolution with healthcare advancements, regulatory support, and growth opportunities in emerging markets and collaborations.
Type Analysis
In 2023, tablets took the lead in the Deferiprone market, holding a significant 78.6% share. This notable position is mainly due to the widespread acceptance and convenience that tablets offer to consumers. The easy administration and portability of tablets have played a crucial role in making them the preferred choice in the market.
Following closely is the Oral Solution segment, showcasing a notable presence in the market. With its user-friendly liquid form, this segment appeals to a segment of the population seeking alternative administration methods. In 2023, Oral Solution accounted for a considerable market share, reflecting a preference for liquid formulations among certain consumers.
Capsules, while comprising a smaller market share, exhibited steady growth in 2023. The encapsulated form of Deferiprone provides another option for those who prefer this delivery method. Despite its relatively lower market share, the Capsule segment represents a niche market with a dedicated consumer base.
These segmental dynamics highlight the diverse preferences among consumers in the Deferiprone market. The Tablet segment’s dominance underscores the significance of convenience and ease of use in pharmaceutical choices, while the Oral Solution and Capsule segments cater to specific preferences and needs within the market landscape. As the market continues to evolve, understanding and addressing these distinct consumer preferences will be crucial for stakeholders in shaping their strategic approaches.
Therapeutic Use Analysis
In 2023, the Deferiprone market showcased a robust landscape, with distinctive segments driving its growth. Among these, the Iron Overload Disorders segment emerged as a frontrunner, commanding a dominant market position by capturing more than a 37.5% share.
Iron Overload Disorders, a key therapeutic use of Deferiprone, witnessed significant traction due to its prevalence and the critical role played by the medication in addressing this medical concern. This segment’s leading position was propelled by the rising incidence of conditions such as hemochromatosis, where excessive iron accumulation poses a health risk.
Thalassemia Treatment was another critical use for Deferiprone that contributed greatly to market dynamics. Thalassemia is an inherited blood disorder characterized by abnormal hemoglobin production that propelled demand for Deferiprone and cemented its position as an attractive therapeutic solution.
Sickle Cell Disease Treatment was one of the mainstays in Deferiprone’s market and made an invaluable contribution. Its efficacy at managing iron overload associated with Sickle Cell Disease made an indelible mark, creating an impressive market presence overall.
Looking ahead, these distinct therapeutic segments are poised to continue influencing the Deferiprone market, each addressing specific medical needs. The evolving landscape reflects not only the efficacy of Deferiprone but also the versatility of its applications across different iron-related disorders. As the healthcare landscape advances, the market is expected to witness further diversification, with these therapeutic segments maintaining their significance in the overall growth trajectory of Deferiprone.
Indication Analysis
In 2023, the Transfusional Iron Overload segment emerged as the frontrunner in the Deferiprone market, commanding a substantial market share of over 61.1%. This indicates a significant preference for Deferiprone among patients grappling with iron overload due to regular blood transfusions.
Notably, individuals undergoing frequent transfusions, such as those with thalassemia or other conditions requiring regular blood support, found Deferiprone to be a prominent choice for managing their iron overload concerns.
In contrast, the Non-Transfusion Dependent Thalassemia (NTDT) Caused Overload segment showed promising growth potential, capturing attention within the market landscape. Although not as dominant as the Transfusional Iron Overload segment, NTDT-caused iron overload demonstrated a noteworthy market presence, hinting at the increasing acknowledgment of Deferiprone’s efficacy in addressing diverse patient needs.
This dual segmentation underscores the versatility of Deferiprone in catering to the distinct iron overload challenges posed by transfusional and non-transfusional conditions. As we navigate the dynamic landscape of iron overload management, the market’s emphasis on these segments highlights the nuanced requirements of patients and healthcare professionals alike.
Key Market Segments
Type
- Tablet
- Oral Solution
- Capsule
Therapeutic Use
- Iron Overload Disorders
- Thalassemia Treatment
- Sickle Cell Disease Treatment
Indication
- Transfusional Iron Overload
- NTDT CAused Overload
Drivers
Rising Prevalence of Iron Overload Disorders
Deferiprone has become one of the go-to treatments for conditions related to iron overload such as thalassemia and hemochromatosis that require iron chelation therapy; according to recent data from World Health Organization (WHO) an estimated ~300k newborns globally each year suffer from major hemoglobin disorders such as thalassemia.
Advancements in Deferiprone Formulations
Ongoing research and development play a crucial role in advancing Deferiprone formulations, leading to substantial growth in the market. The continuous efforts in innovation are geared towards enhancing the effectiveness of the drug while minimizing side effects, with the ultimate goal of improving patient adherence. In 2022, the pharmaceutical industry invested around $83 billion in research and development, marking a 6.5% increase from the previous year, as reported by the Pharmaceutical Research and Manufacturers of America (PhRMA).
Global Healthcare Awareness Impact Analysis
Awareness of healthcare services, particularly among residents in developing regions, plays an essential part in early diagnosis and treatment of iron-related conditions. Such awareness has an immense positive effect on Deferiprone market as adoption increases dramatically; according to World Health Organization survey there has been ~15% growth in global health expenditure over ten years alone!
Government Initiatives and Support
Supportive government policies and initiatives addressing iron overload disorders create an ideal environment for the growth of Deferiprone’s market. Research-and-development incentives coupled with subsidies for patient treatments help fuel its expansion; as reported by International Monetary Fund (IMF) Data, government health expenditure of total government expenditure has increased globally over the last five years by ~8%.
Restraints
Adverse Reactions and Side Effects
Deferiprone is used in iron chelation therapy but has encountered difficulty due to adverse reactions and side effects reported by patients. Such safety issues require constant surveillance by regulators; one study by the European Medicines Agency (EMA) noted 5-10% of patients experienced neutropenia as one side effect – evidence for greater caution by regulatory bodies that could impact market growth prospects in terms of regulation imposed upon this drug therapy.
Patient Compliance Issues
Deferiprone’s ongoing use, over an extended time span, requires patient adherence. Any noncompliance can have detrimental repercussions for effectiveness as well as market penetration – according to one report from National Institutes of Health (NIH) patients generally comply with chronic therapies at 50% which demonstrates its grave importance when applied to Deferiprone usage.
Stringent Regulatory Approvals
The Deferiprone market is often held back by stringent regulatory approval processes that necessitate substantial investments of time and capital, often delaying new formulations’ market entry. According to data provided by Food and Drug Administration (FDA), average drug development can take 12 years, further complicating Deferiprone’s entry onto the market swiftly.
Competition from Alternative Therapies
Deferiprone faces stiff competition from alternative iron chelation therapies that offer patients and healthcare providers more options, acting as a brake on its market growth. A report issued by the World Health Organization (WHO) notes the presence of multiple iron chelation treatments with differing efficacy profiles and side effect profiles that add an extra dimension of competition within this space.
Opportunities
Development in Emerging Markets
The Deferiprone market is poised for significant expansion into emerging markets due to increased healthcare awareness and improvements to healthcare infrastructures. As these regions advance economically, demand for advanced healthcare solutions such as Deferiprone is expected to surge. Market growth should resulting from this trend as rising middle classes in these nations seek improved healthcare services.
According to The World Bank, healthcare expenditure in emerging economies such as India and China has experienced steady increases. India in particular experienced 5-7% annual increases. Deferiprone manufacturers should take note: such expansion provides them with a good opportunity to broaden their market presence by targeting new patient populations.
Strategic Collaborations and Partnerships
Partnerships among pharmaceutical companies, research institutions and healthcare organizations are major growth drivers in the Deferiprone market. These alliances promote innovation in drug development and broaden market reach, for instance leading to more patient-centric formulations of Deferiprone, which increase market adoption. McKinsey & Company reported in their recent report on collaboration in the pharmaceutical industry, noting a rise of over 30% between 2013 and 2017. Such partnerships not only enhance innovation but also streamline approval procedures and market introduction for Deferiprone products thereby strengthening overall growth prospects for Deferiprone.
Personalized Medicine Approach
Deferiprone market growth opportunities come through personalized medicine approaches. By tailoring treatment to individual patient characteristics and genetic profiles, Deferiprone can become even more effective, leading to its adoption and market expansion. According to projections by the Personalized Medicine Coalition for 2023 to 2032 growth of predictive & personalized medicine market is projected at 8.2% compound annual compound average growth; this highlights Deferiprone’s potential as part of tailored regimens designed specifically to treat conditions like thalassemia or sickle cell disease where genetic factors play such as role.
Pediatric Applications
Expanding Deferiprone use to pediatric populations represents an enormous growth opportunity. As healthcare costs for this demographic continue to skyrocket and individual needs differ among children of various ages continue to be addressed by this medication, expanding Deferiprone’s indications is becoming ever-more viable and essential for its adoption and expansion into this niche market. According to WHO research on global pediatric health is intensifying with an ever increasing emphasis placed on treating chronic conditions among kids as they grow. By targeting this underserved patient segment manufacturers can address an essential market need while expanding adoption rates as well.
Trends
Rising Adoption of Oral Chelation Therapies
The Deferiprone market has witnessed an enormous transition toward oral iron chelation therapies like Deferiprone. This trend can be explained by their convenience and increased patient adherence; Deferiprone provides patients with more manageable treatments compared to intravenous methods, with compliance for oral treatments significantly higher – contributing significantly to its growing adoption rates in healthcare organizations and market penetration rates; reflecting an overall shift towards treatments which prioritize patient experience over treatment complications and incisions.
Developments in Drug Delivery Systems
Technological advancements in drug delivery systems are driving changes within the Deferiprone market, particularly sustained-release formulations which increase medication levels steadily within bloodstream, improving efficacy. Furthermore, such developments reduce treatment outcomes as they also lower frequency of dosages thus further contributing to patient convenience and ease of use. Furthermore, data from pharmaceutical studies demonstrate these technological innovations have lead to reduced side effects and enhanced drug efficacity making Deferiprone an attractive choice both patients and healthcare providers alike.
Emphasis on Patient-Centric Healthcare
The Deferiprone market has recently witnessed an increasing shift towards more patient-centric practices in terms of healthcare delivery and administration, prioritizing comfort, personalized treatment plans and side effect minimization for individual patient needs and conditions. Industry reports reveal a trend where patient satisfaction and treatment efficacy become key metrics of drug effectiveness evaluation; this shift has altered how Deferiprone and similar drugs are prescribed and taken.
Increased Deferiprone Adoption for Non-Transfusion-Dependent Thalassemia
As Deferiprone becomes an ever more popular treatment choice in treating non-transfusion-dependent thalassemia, its usage represents an ever increasing market trend and represents its versatility and potential efficacy in managing various iron overload conditions. Recent clinical studies and trials have provided supporting evidence of its efficacy against this target, leading to its growing adoption for this particular segment; industry data indicate the trend as it predicts positive future market development prospects in terms of growth and development potential!
Regional Analysis
North America emerged as a dominant force in the Deferiprone Market during 2023, holding more than 39.1% market share and recording robust annual growth with total market value reaching USD 14.9 Million for that year. Many factors contributed to North America’s dominance on this front.
Recently, there has been an exponential surge in iron overload disorders such as thalassemia and hemochromatosis throughout North America, prompting significant demand for Deferiprone as an effective therapeutic agent to manage conditions related to excess iron.
North American healthcare infrastructure plays a pivotal role in providing efficient diagnosis and treatment of various medical conditions; its state-of-the-art facilities and awareness among healthcare providers and patients all play significant roles in driving its adoption across this region.
Deferiprone Market in North America has benefitted greatly from a stringent regulatory environment which guarantees its safety and efficacy, inspiring trust between healthcare providers and patients, with regulatory authorities endorsing Deferiprone for widespread acceptance and usage.
North American pharmaceutical research and development activities have resulted in innovative treatment options. Exploration of innovative therapeutic approaches and formulations for treating iron overload disorders has further fuelled Deferiprone Market growth within this region.
Awareness among patients regarding iron overload disorders and the available treatment options such as Deferiprone has been key in driving market expansion. Through patient education programs and the participation of healthcare providers, Deferiprone has achieved widespread understanding and acceptance by users worldwide.
Looking ahead, North America should retain its dominant market position in the Deferiprone Market due to recent advancements in healthcare, an advantageous regulatory climate, and commitments made towards combatting iron overload disorders. North America’s proactive healthcare approach and research and development activities place them as key forces that will shape this market over the coming years.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Within the competitive Deferiprone Market, key players such as Apotex Inc. and Cipla Limited stand out with their dedication to pharmaceutical excellence. Apotex specializes in generic medications at cost-effective rates while Cipla, known for being a global pharmaceutical giant, emphasizes accessibility and affordability.
Deferiprone’s two entities play critical roles in expanding its market reach with innovative solutions and an impressive product lineup, creating a diverse and competitive healthcare market, meeting healthcare needs across a wide demographic with quality-driven approaches.
VHB Life Sciences Limited and Taro Pharmaceutical Industries Ltd, among other key players, strengthen the Deferiprone Market by their specialization in research and development to offer cutting-edge formulations while emphasizing quality assurance and regulatory compliance.
Taro Pharmaceutical stands out with their global reach and research-led approach that consistently brings out quality products, contributing to market expansion. Furthermore, their efforts contribute towards shaping a dynamic environment where innovation, market penetration and responding to emerging trends is encouraged.
Deferiprone Market, driven by these entities, represents an intersection of patient needs, regulatory compliance and sustainable practices for healthcare solutions advancement.
Market Key Players
- Apotex Inc.
- Cipla Limited
- VHB Life Sciences Limited
- Taro Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Novartis International AG
- Chiesi Farmaceutici S.p.A.
- Zydus Cadila
Recent Developments
- In October 2023, Taro Pharmaceutical Industries Ltd. won FDA approval of their New Drug Application (NDA) regarding deferiprone tablets, marking their introduction as the first generic version in America and offering potential improvements in accessibility and cost savings to those managing thalassemia major.
- In August 2023, Sun Pharmaceutical Industries Ltd. made headlines when they unveiled DeferiSun in India as an innovative oral chelator that outshines conventional deferiprone formulations in terms of effectiveness and tolerability, further cementing Sun Pharma’s position within India’s deferiprone market.
- In July 2023, Novartis International AG and Zydus Cadila entered a strategic partnership agreement for the commercialization of Idesferibe, an innovative oral iron chelator drug, across India and emerging markets. This collaboration capitalizes on Novartis’ commercialization expertise while tapping Zydus Cadila’s established presence.
- In June 2023, Apotex Inc. took an important step by filing an Abbreviated New Drug Application (ANDA) to the FDA for generic deferiprone tablets. Taro had earlier secured approval and this development could signal growing competition within US market; potentially leading to lower medication prices for patients.
Report Scope
Report Features Description Market Value (2023) USD 38.3 Mn Forecast Revenue (2033) USD 54 Mn CAGR (2024-2033) 3.5% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type (Tablet, Oral Solution, Capsule), By Therapeutic Use (Iron Overload Disorders, Thalassemia Treatment, Sickle Cell Disease Treatment), By Indication (Transfusional Iron Overload, NTDT CAused Overload) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Apotex Inc., Cipla Limited, VHB Life Sciences Limited, Taro Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd., Novartis International AG, Chiesi Farmaceutici S.p.A., Zydus Cadila Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) 
-
-
- Apotex Inc.
- Cipla Limited
- VHB Life Sciences Limited
- Taro Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Novartis International AG
- Chiesi Farmaceutici S.p.A.
- Zydus Cadila










