Global Cryptococcal Antigen Lateral Flow Assay Test Market By Instruments (Lateral Flow Readers and Kits & Reagents), By Test Location (Home Testing, Point of Care Testing, and Laboratory Testing), By End-user (Hospitals, Ambulatory Clinics, Diagnostic Laboratories, and Home Healthcare), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Dec 2025
- Report ID: 170076
- Number of Pages: 310
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
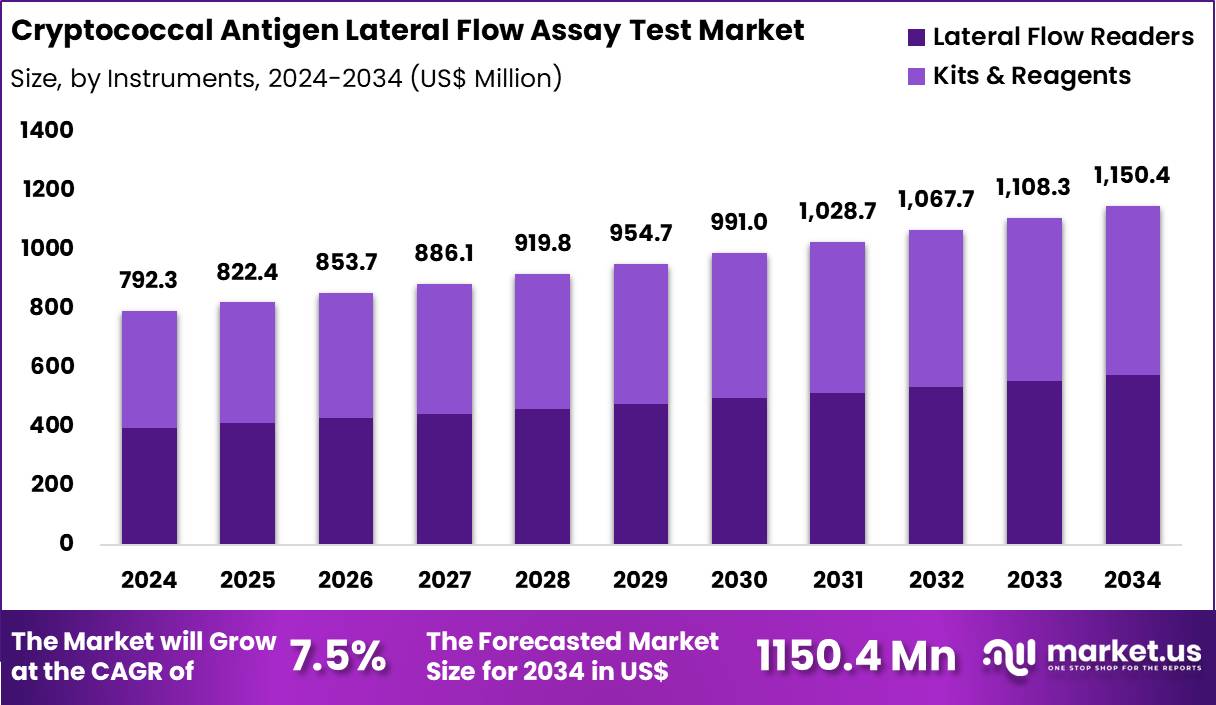
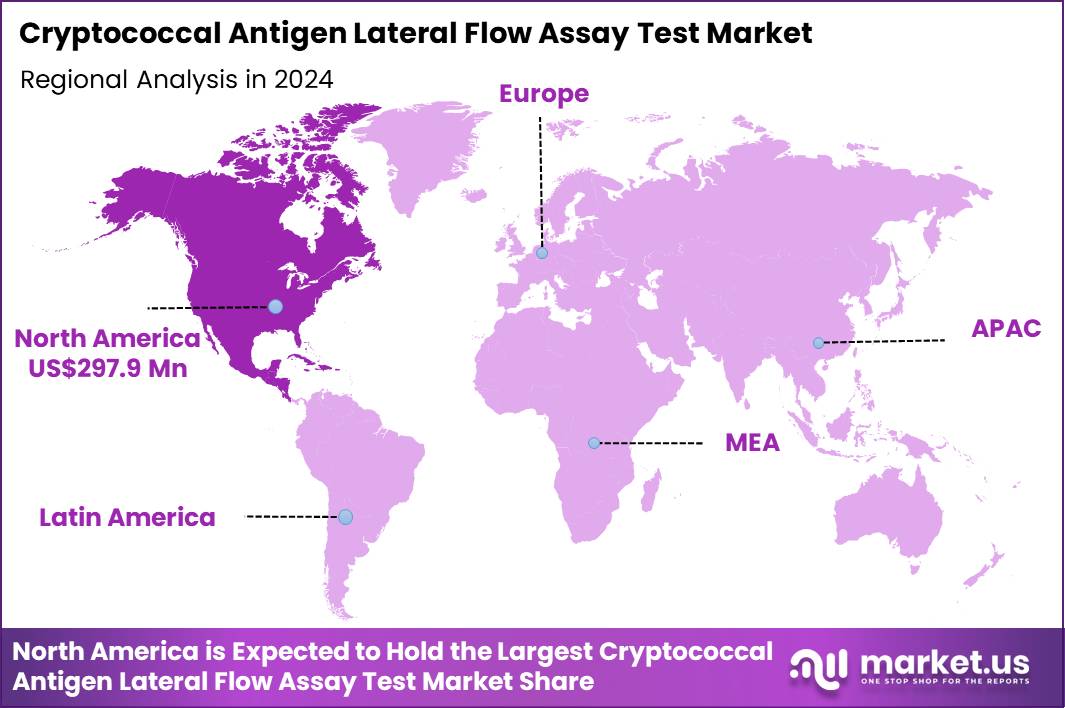
The Global Cryptococcal Antigen Lateral Flow Assay Test Market size is expected to be worth around US$ 1150.4 Million by 2034 from US$ 792.3 Million in 2024, growing at a CAGR of 3.8% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 37.6% share with a revenue of US$ 297.9 Million.
Increasing incidence of opportunistic fungal infections in immunocompromised patients propels the Cryptococcal Antigen Lateral Flow Assay Test market, as clinicians prioritize rapid diagnostics to initiate timely antifungal interventions and reduce mortality risks. Diagnostic firms enhance lateral flow platforms with monoclonal antibodies that achieve superior sensitivity for detecting capsular polysaccharides in serum, plasma, and cerebrospinal fluid samples.
These tests enable early screening in HIV-positive individuals to preempt cryptococcal meningitis progression, confirmatory diagnosis in febrile neutropenia cases for immediate amphotericin B dosing, and therapeutic monitoring in transplant recipients to assess antigen clearance during fluconazole maintenance. Multiplex innovations create opportunities for bundled assays that streamline workflows in resource-constrained settings.
In March 2024, prominent diagnostic companies including IMMY and Meridian Bioscience, now integrated into Revvity, advanced multiplex lateral flow capabilities to detect cryptococcal antigen alongside HIV antibodies, markedly broadening point-of-care utility for integrated infection management. This development elevates clinical efficiency and positions the market for accelerated adoption in high-burden environments.
Growing emphasis on point-of-care accessibility accelerates the Cryptococcal Antigen Lateral Flow Assay Test market, as healthcare providers deploy user-friendly dipstick formats that deliver results in under 10 minutes without specialized equipment. Biotechnology companies refine semiquantitative scoring systems that correlate band intensity with disease burden for prognostic insights.
Applications encompass outpatient HIV clinic reflex testing to guide preemptive therapy in asymptomatic carriers, emergency department evaluation of headache and fever in endemic areas, pediatric meningitis differentiation from bacterial etiologies, and veterinary diagnostics for avian cryptococcosis in exotic pet care.
Decentralized testing opens avenues for community health worker training and mobile outreach programs that enhance equity in fungal disease detection. Pharmaceutical developers increasingly incorporate these assays into clinical trials for novel azole formulations, validating endpoints for regulatory submissions. This portability trend drives sustained innovation in stable, ambient-temperature reagents.
Rising integration of digital result interpretation invigorates the Cryptococcal Antigen Lateral Flow Assay Test market, as laboratories adopt smartphone-linked readers that quantify antigen levels and generate automated reports for electronic health records. Technology providers embed AI algorithms into companion apps that flag indeterminate results and recommend confirmatory cultures. These enhancements support outbreak surveillance in long-term care facilities for environmental exposure tracking, research applications in vaccine efficacy studies against C.
gattii strains, and global health initiatives for mass screening in tuberculosis-HIV co-infection cohorts. Quantitative capabilities create opportunities for longitudinal studies assessing antifungal resistance emergence and treatment adherence. Collaborative consortia actively standardize digital protocols to facilitate data sharing across networks, bolstering epidemiological modeling. This digital evolution establishes lateral flow assays as versatile pillars in modern mycology diagnostics.
Key Takeaways
- In 2024, the market generated a revenue of US$ 792.3 Million, with a CAGR of 3.8%, and is expected to reach US$ 1150.4 Million by the year 2034.
- The instruments segment is divided into lateral flow readers and kits & reagents, with lateral flow readers taking the lead in 2024 with a market share of 50.2%.
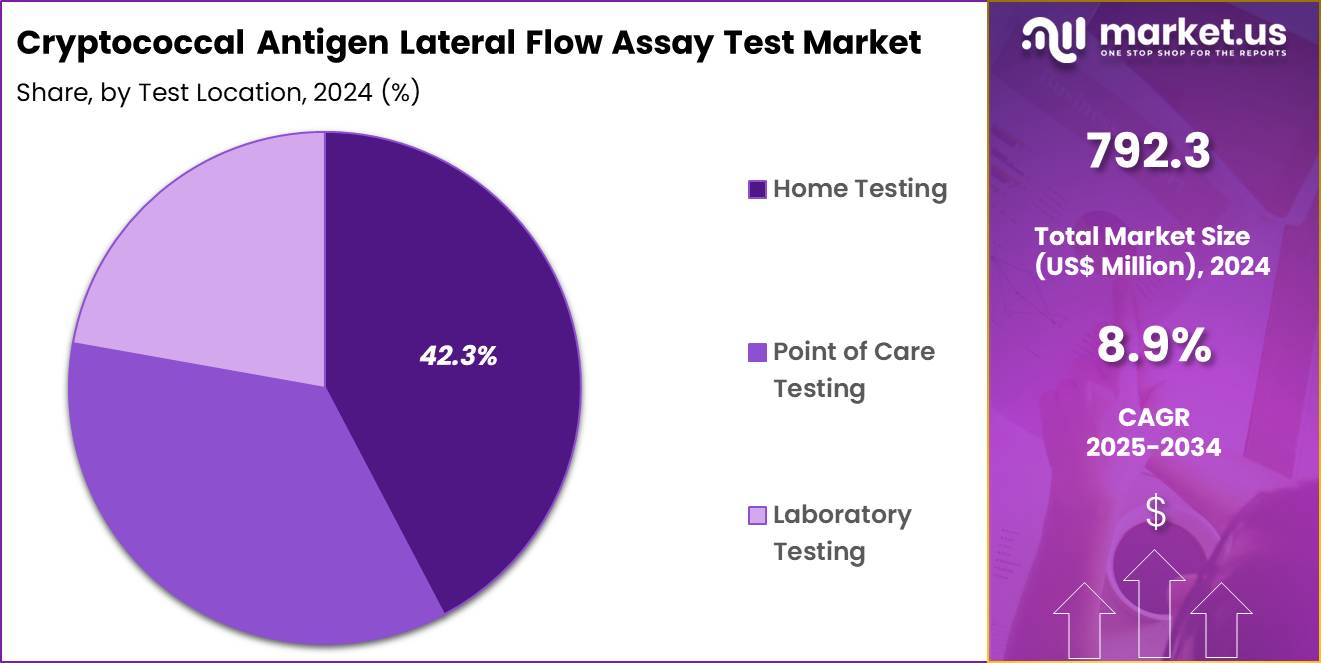
- Considering test location, the market is divided into home testing, point of care testing, laboratory testing. Among these, home testing held a significant share of 42.3%.
- Furthermore, concerning the end-user segment, the market is segregated into hospitals, ambulatory clinics, diagnostic laboratories, home healthcare. The hospitals sector stands out as the dominant player, holding the largest revenue share of 46.9% in the market.
- North America led the market by securing a market share of 37.6% in 2024.
Instruments Analysis
Lateral Flow Readers, holding 50.2%, are expected to dominate due to their ability to deliver quick, accurate results for cryptococcal antigen testing at various testing points. The demand for lateral flow readers is driven by their widespread use in point-of-care and home testing settings, where rapid diagnosis is crucial for managing cryptococcal infections. These readers offer ease of use, portability, and automation, improving workflow efficiency in hospitals, clinics, and at home.
Advances in reader technology continue to improve sensitivity and precision, making them more reliable for both healthcare professionals and patients. Increased accessibility to healthcare services and the need for faster diagnostic methods in resource-limited areas further strengthen the adoption of lateral flow readers. These factors keep lateral flow readers projected to remain the dominant product in the cryptococcal antigen lateral flow assay test market.
Test Location Analysis
Home testing, holding 42.3%, is anticipated to grow rapidly as consumers increasingly seek convenient, at-home diagnostic options for early detection of fungal infections like cryptococcal meningitis. The growing acceptance of at-home testing devices is largely driven by the ease of use and privacy they offer, allowing individuals to perform tests in the comfort of their homes. Healthcare providers also recognize the importance of empowering patients with self-testing tools, especially in regions with limited access to healthcare facilities.
The increasing number of chronic disease cases, particularly among immunocompromised individuals, strengthens the need for home testing. Advancements in test kit design and the availability of digital platforms for result interpretation further support this growth. These trends keep home testing expected to remain one of the most dominant testing locations in the cryptococcal antigen lateral flow assay test market.
End-User Analysis
Hospitals, holding 46.9%, are expected to remain the dominant end-user segment due to their central role in providing comprehensive healthcare, including diagnosing and treating serious infections like cryptococcosis. Hospitals require high-volume testing solutions and rely heavily on lateral flow assays to ensure quick results, particularly in critical care and emergency departments. The prevalence of fungal infections in immunocompromised patients, such as those with HIV/AIDS or undergoing chemotherapy, strengthens demand for early detection.
Hospitals also benefit from integrating lateral flow testing into their diagnostic workflows, improving patient outcomes by enabling prompt treatment. With an expanding global focus on improving diagnostic capabilities in hospitals, the demand for efficient, rapid testing methods, like lateral flow assays, will continue to grow. These factors ensure hospitals remain the leading end-user in the cryptococcal antigen lateral flow assay test market.
Key Market Segments
By Instruments
- Lateral Flow Readers
- Kits & Reagents
By Test Location
- Home Testing
- Point of Care Testing
- Laboratory Testing
By End-user
- Hospitals
- Ambulatory Clinics
- Diagnostic Laboratories
- Home Healthcare
Drivers
The High Global Burden of HIV-Associated Cryptococcosis Is Driving the Market
The high global burden of HIV-associated cryptococcosis serves as a fundamental driver for the cryptococcal antigen lateral flow assay test market, as it amplifies the need for rapid, scalable diagnostics in resource-limited settings. This opportunistic infection primarily affects individuals with advanced HIV disease, where weakened immunity facilitates fungal dissemination, often leading to life-threatening meningitis. Health organizations worldwide prioritize antigen detection to enable preemptive therapy, reducing mortality through early intervention.
In regions with suboptimal antiretroviral coverage, cryptococcal disease accounts for a substantial proportion of AIDS-related fatalities, necessitating widespread screening protocols. The Centers for Disease Control and Prevention estimates that cryptococcal meningitis causes more than 100,000 HIV-related deaths annually. Such prevalence underscores the assay’s role in integrating into routine CD4 monitoring workflows for at-risk populations.
Manufacturers are scaling production to meet demands from national programs in sub-Saharan Africa and Southeast Asia, where incidence peaks. Economic models demonstrate that point-of-care testing averts costly hospitalizations, justifying investments in assay distribution. As global HIV initiatives expand, the market benefits from aligned procurement frameworks that prioritize accessible diagnostics. This driver positions the lateral flow assay as a cornerstone for equitable fungal infection control.
Restraints
Limited Integration into Routine Non-HIV Immunocompromised Screening Protocols Is Restraining the Market
Limited integration into routine screening protocols for non-HIV immunocompromised patients restrains the cryptococcal antigen lateral flow assay test market by confining its application to HIV-centric guidelines, despite rising cases in transplant and oncology cohorts. While established in HIV care, adoption lags in solid organ transplant programs due to variable clinician awareness and absence of universal recommendations. This oversight results in delayed diagnoses, as symptoms mimic other etiologies in these populations, complicating empiric management.
Resource allocation favors high-prevalence HIV settings, sidelining broader implementation amid competing diagnostic priorities. The Centers for Disease Control and Prevention notes that cryptococcosis prevalence reached 3.4 cases per 100,000 among commercially insured patients from 2016 to 2022, with higher rates in Medicaid populations, yet screening remains inconsistent outside AIDS contexts. Logistical challenges, including assay storage and training, further hinder uptake in specialized wards.
Reimbursement disparities across payers discourage routine use, perpetuating reliance on culture-based confirmations. Educational gaps exacerbate hesitancy, as providers undervalue antigen testing’s sensitivity in non-meningeal disease. Consequently, preventable complications persist, undermining market expansion in diverse clinical niches. Addressing this restraint involves guideline harmonization to encompass emerging risk groups.
Opportunities
Expansion of Point-of-Care Diagnostics in Low-Resource Settings Is Creating Growth Opportunities
Expansion of point-of-care diagnostics in low-resource settings creates substantial growth opportunities for the cryptococcal antigen lateral flow assay test market by bridging gaps in laboratory infrastructure and enabling decentralized screening. These environments, burdened by high HIV loads, benefit from assays requiring minimal equipment, facilitating community-level implementation. Partnerships with global health entities accelerate distribution, aligning with sustainable development goals for infectious disease control.
Opportunities emerge from bundling with HIV rapid tests, streamlining workflows in primary care clinics. Validation studies confirm the assay’s 99% pooled sensitivity and specificity in cerebrospinal fluid among HIV-negative patients, supporting versatile applications. Integration into mobile health units extends reach to remote areas, enhancing equity in access. Cost-effectiveness analyses reveal savings through averted advanced disease treatment, attracting donor funding for scale-up.
Emerging markets in Latin America and Eastern Europe present untapped potential, where rising immunosuppression trends demand robust tools. Training modules for non-specialists further democratize usage, fostering local capacity. Collectively, these opportunities propel the market toward inclusive, high-impact diagnostic ecosystems.
Impact of Macroeconomic / Geopolitical Factors
Economic growth and surging healthcare investments accelerate the cryptococcal antigen lateral flow assay test market, as providers deploy rapid diagnostics to detect fungal infections in immunocompromised patients, especially those with HIV/AIDS in high-burden regions. Inflation, however, inflates costs for reagents and assay components, which pressures manufacturers to raise prices and limits access in low-resource clinics across Africa and Asia.
Geopolitical tensions, notably U.S.-China trade barriers, disrupt supply chains for imported testing materials from Asia, which delays availability and escalates shipping fees for global distributors. These tensions, on the positive side, prompt governments in Europe and Latin America to fund local biotech facilities, which fosters innovation and reduces exposure to international disruptions. Current U.S. tariffs impose a 10% baseline duty on medical diagnostics alongside up to 25% on devices from select partners effective April 2025, which burdens importers and hikes expenses for U.S. hospitals reliant on foreign kits.
Yet, these tariffs encourage American firms to expand domestic production, which creates jobs and ensures more stable supplies amid global uncertainties. Despite these challenges, escalating needs for point-of-care testing sustain market momentum. Ultimately, technological refinements and global health partnerships position the cryptococcal antigen lateral flow assay test sector for resilient expansion and life-saving impact worldwide.
Latest Trends
The Publication of the Global Guideline for Cryptococcosis Diagnosis in 2024 Is a Recent Trend
The publication of the Global Guideline for the Diagnosis and Management of Cryptococcosis on February 9, 2024, by the European Confederation of Medical Mycology, International Society for Human and Animal Mycology, and American Society for Microbiology represents a pivotal recent trend in standardizing cryptococcal antigen lateral flow assay utilization. This comprehensive document emphasizes the assay’s role as the preferred initial test for suspected cryptococcal disease across diverse hosts, including HIV and non-HIV cases.
It advocates for serum screening in advanced HIV with CD4 counts below 200 cells per microliter, integrating lateral flow results into risk-stratified algorithms. The guideline highlights the assay’s superior performance over traditional methods, with recommendations for semiquantitative interpretation to guide therapy intensity. Endorsed by major infectious disease bodies, it influences national protocols, particularly in endemic regions.
Key sections address implementation barriers, promoting point-of-care adoption to reduce diagnostic delays. This trend coincides with heightened focus on fungal priorities, as designated by the World Health Organization in 2022. Early endorsements from clinical networks signal accelerated guideline dissemination through educational webinars. As adoption surges, it enhances assay demand in both ambulatory and inpatient settings. Overall, this 2024 advancement reinforces evidence-based practices for optimal patient outcomes.
Regional Analysis
North America is leading the Cryptococcal Antigen Lateral Flow Assay Test Market
North America accounted for 37.6% of the overall market in 2024, and the region experienced significant growth as healthcare systems increasingly adopted rapid, point-of-care diagnostics for cryptococcal infections, particularly in immunocompromised patients. Hospitals and clinical labs expanded the use of Cryptococcal Antigen Lateral Flow Assay Tests to quickly diagnose meningitis caused by Cryptococcus species in individuals with HIV/AIDS, organ transplant recipients, and patients undergoing immunosuppressive treatments.
These tests gained popularity due to their ease of use, low cost, and quick results, providing clinicians with valuable information for early intervention. The Centers for Disease Control and Prevention (CDC) estimated 1 in 5 people with HIV in the U.S. are affected by cryptococcal meningitis annually (CDC – “Cryptococcal Meningitis in HIV Infected Persons 2023”), driving demand for efficient and rapid diagnostic solutions. The growing prevalence of fungal infections in at-risk populations further fueled the adoption of these tests. These factors contributed to strong market growth in North America.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to see steady growth during the forecast period as healthcare systems in developing regions strengthen diagnostic capabilities to combat infectious diseases like cryptococcosis. The increasing prevalence of HIV/AIDS, coupled with growing awareness of fungal infections, drives the demand for rapid diagnostic tools in hospitals and healthcare facilities. Public-health organizations push for greater access to affordable testing, particularly in rural and underserved areas where cryptococcal infections are often underdiagnosed.
The World Health Organization (WHO) reported that 15 million people in Asia Pacific are living with HIV, many of whom are at higher risk for cryptococcal infections (WHO – “HIV/AIDS in Asia and the Pacific 2023”). This significant burden of disease increases the need for early and accurate detection methods, positioning cryptococcal antigen testing as a critical tool in managing the health of at-risk populations. As healthcare infrastructure continues to improve, the market for rapid diagnostic tests is expected to grow.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key firms in the cryptococcal‑antigen lateral flow assay (CrAg LFA) market drive growth by enhancing test sensitivity and reducing time‑to‑result, enabling early detection of invasive fungal infections in immunocompromised patients a factor that boosts adoption in hospitals and clinics that manage high HIV or transplant caseloads. They expand their geographic reach by partnering with regional public‑health bodies and diagnostic lab networks in low‑resource settings, ensuring supply of affordable, easy‑to‑use kits.
They invest in R&D to improve assay stability and broaden sample‑type compatibility (serum, plasma, CSF, whole blood), allowing wider clinical application and easier administration. They integrate CrAg assays into multiplex or broader infectious‑disease screening panels to increase value per sample and appeal to high‑volume laboratories.
They secure long‑term revenue by aligning with global health‑care initiatives targeting opportunistic infections and by forging collaborations with NGOs and governmental programs to scale screening. One of the prominent players, IMMY manufactures the CrAg LFA strip, leverages its specialized focus on mycology diagnostics, maintains regulatory approvals, and uses its distribution network and strong assay‑performance reputation to supply reliable cryptococcal antigen screening solutions worldwide.
Top Key Players
- bioMérieux SA
- Danaher Corporation
- Qiagen N.V.
- Roche Diagnostics
- Merck Millipore (Merck KGaA)
- Alere, Inc.
- Kestrel Biosciences
- Forsite Diagnostics
Recent Developments
- In April 2025, IMMY (Immuno-Mycologics, Inc.) boosted its production capacity for the CrAg LFA Test to meet the growing global demand, particularly in regions affected by HIV/AIDS where cryptococcal meningitis remains a significant concern. This expansion aligns with ongoing public health efforts to address fungal infections in vulnerable populations.
- On February 28, 2024, bioMérieux formed a strategic partnership with the U.S. FDA’s Center for Food Safety and Applied Nutrition (CFSAN) to advance the development of diagnostic solutions for detecting foodborne pathogens such as Salmonella and E. coli. This collaboration enhances bioMérieux’s lateral flow assay (LFA) technology, which will support their broader infectious disease diagnostics portfolio, including future innovations in CrAg testing.
Report Scope
Report Features Description Market Value (2024) US$ 792.3 Million Forecast Revenue (2034) US$ 1150.4 Million CAGR (2025-2034) 3.8% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Instruments (Lateral Flow Readers and Kits & Reagents), By Test Location (Home Testing, Point of Care Testing, and Laboratory Testing), By End-user (Hospitals, Ambulatory Clinics, Diagnostic Laboratories, and Home Healthcare) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape bioMérieux SA, Danaher Corporation, Qiagen N.V., Roche Diagnostics, Merck Millipore (Merck KGaA), Alere, Inc., Kestrel Biosciences, Forsite Diagnostics Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Cryptococcal Antigen Lateral Flow Assay Test MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample
Cryptococcal Antigen Lateral Flow Assay Test MarketPublished date: Dec 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- bioMérieux SA
- Danaher Corporation
- Qiagen N.V.
- Roche Diagnostics
- Merck Millipore (Merck KGaA)
- Alere, Inc.
- Kestrel Biosciences
- Forsite Diagnostics










