Global Amyotrophic Lateral Sclerosis Treatment Market, By Type (Sporadic ALS and Familial ALS), By Drug Type (Riluzole, Edaravone, and Nuedexta), By Treatment (Medication, Respiratory Therapy, Speech Therapy, Physical Therapy, Chemotherapy, Stem Cell Therapy, and Other Treatments), By End Users (Hospitals, Speciality Centres, Research and Academic Institutes, Diagnostic Centres, and Other End-Users), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 49399
- Number of Pages: 295
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
The Global Amyotrophic Lateral Sclerosis Treatment Market was valued at USD 600 Million in 2022 and expected to grow USD 1021 Million in 2032. Between 2023 and 2032, this market is estimated to register a CAGR of 5.6%.
The global amyotrophic lateral sclerosis (ALS) treatment market refers to the market for pharmaceutical drugs, medical devices, and other therapies used for the treatment of ALS, a progressive and ultimately fatal neurological disorder that affects the nerve cells in the brain and spinal cord. ALS leads to the degeneration and death of motor neurons, which control muscle movement, leading to muscle weakness, paralysis, and eventually, respiratory failure.
The ALS treatment market includes FDA-approved drugs such as riluzole and edaravone, which are used to slow the progression of the disease, as well as medical devices such as ventilators, feeding tubes, and mobility aids, which are used to support individuals with ALS, as the disease progresses. The market is driven by an increasing incidence of the disease, advancements in medical technology and drug development, and a growing awareness of the need for effective treatments for ALS.
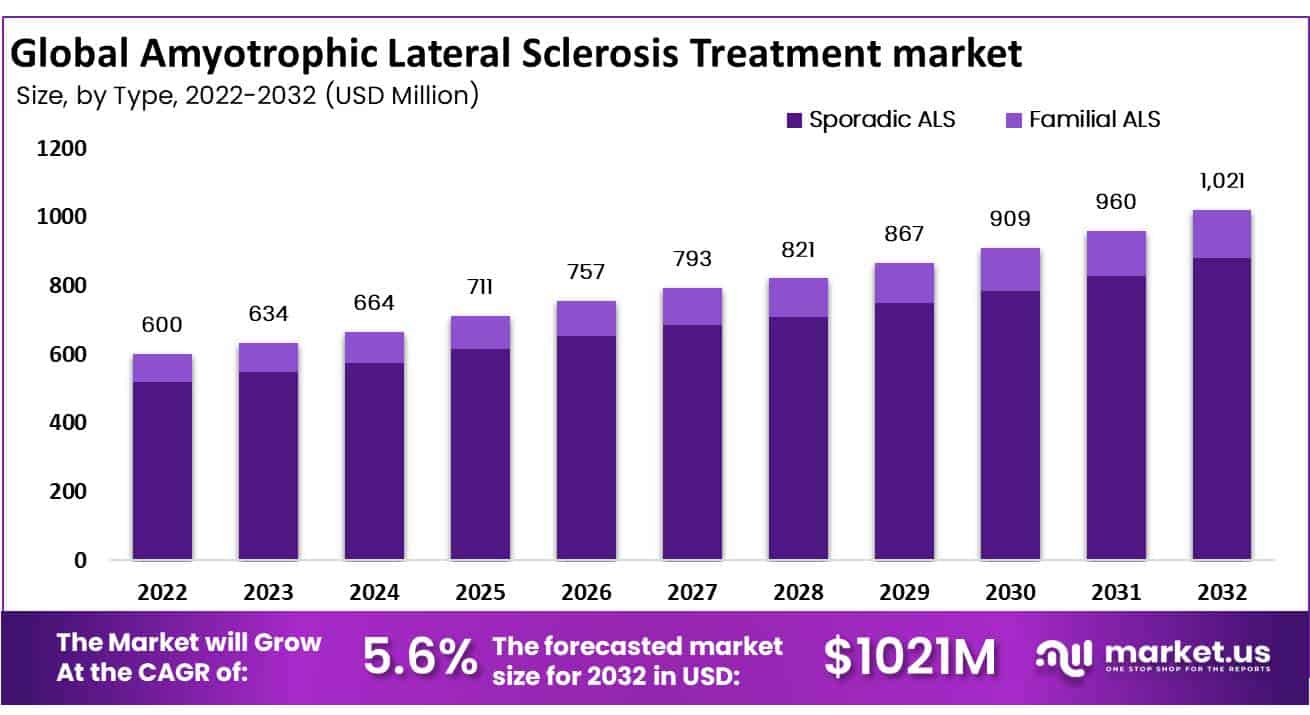
*Actual Numbers Might Vary In The Final Report
Key Takeaways
- High Unmet Medical Need: Due to no available cure for ALS and available therapies that focus mainly on managing symptoms and improving quality of life for patients, research and development in this area are driving high unmet medical need. This calls for continuous efforts toward finding more treatments.
- Symptomatic Treatments for ALS: Treatment approaches typically focus on relieving symptoms through medication (Riluzole and Edaravone are approved treatments to slow disease progression while managing symptoms) as well as physical therapy, occupational therapy and speech therapy to enhance patient functionality.
- Experimental Therapies and Interventions: At present, numerous experimental therapies and interventions are currently undergoing clinical trials, such as stem cell therapies, gene therapy and neuroprotective agents aimed at targeting the root causes of ALS. Some potential treatments aim to target this condition more directly.
- Development Challenges of Drug Therapies for ALS: Producing effective therapies against ALS presents unique difficulties due to its complexity, rapid progression and the difficulty in finding biomarkers for clinical trials.
- Researching Genetic Factors: Genetic factors may account for up to 25% of cases of ALS. Genetic testing can detect specific gene mutations linked to this illness and therapies aimed at targeting these genetic components are currently under study for personalized treatments.
Driving Factors
Growing Incidence and Prevalence of ALS Disease
ALS is a rare disease, but its incidence and prevalence have been steadily increasing over the years. According to the ALS Association, approximately 5,000 people in the United States are diagnosed with ALS each year. As the population ages, the number of people affected by ALS is expected to rise, which is expected to drive the demand for ALS treatments and therapies.
Advancements in Research and Development
The ALS market has seen several recent advancements in research and development, particularly in the areas of drug discovery and gene therapy. These advancements are expected to lead to the development of new and more effective treatments for ALS patients.
Increasing Investment and Funding
The ALS market has seen increasing investment and funding from governments, non-governmental organizations, and private entities. This investment is expected to support research and development efforts, as well as increase patient access to treatments and therapies.
Growing Awareness and Advocacy
The ALS community has become increasingly vocal and active in raising awareness about the disease and advocating for research and treatment. This has helped to increase public understanding of ALS and generate support for ALS research and development efforts.
Restraining Factors
Lack of Effective Treatments
Currently, there is no cure for ALS, and the available treatments only help to manage symptoms and slow disease progression. This limits the options available to patients and may reduce the overall demand for ALS treatments and therapies.
High Cost of Treatment
ALS treatments and therapies can be expensive, particularly for patients who require ongoing care and management. This can limit patient access to treatment, particularly in low- and middle-income countries.
Regulatory Challenges
The development of new ALS treatments and therapies can be hampered by regulatory challenges, including lengthy approval processes and stringent safety and efficacy requirements. This can delay the availability of new treatments and limit the overall growth of the ALS market.
Growth Opportunities
Development of New Therapies
The development of new and more effective therapies for ALS patients remains a significant opportunity in the market. Research efforts are ongoing in areas such as gene therapy, stem cell therapy, and immunotherapy, which could potentially lead to new treatment options for ALS patients.
Telemedicine and Digital Health Platforms
The use of telemedicine and digital health platforms can provide more accessible and efficient care for ALS patients, particularly those who cannot travel to specialized ALS centers. These platforms can also improve data collection and analysis, which can help accelerate the development of new ALS therapies.
Trending Factors
Gene Therapy
Gene therapy is an emerging trend in the ALS market, with several companies exploring the use of gene therapy to treat ALS. Gene therapy involves delivering healthy genes to replace or supplement faulty genes associated with ALS.
Biomarker Research
There is increasing interest in biomarker research in the ALS market. Biomarkers are measurable indicators of disease that can help with early diagnosis, patient stratification, and treatment monitoring. The identification of reliable ALS biomarkers could significantly improve the diagnosis and management of ALS patients.
Patient-Centered Care
Patient-centered care is a growing trend in healthcare, and it is becoming increasingly important in the ALS market. ALS patients require complex care that is tailored to their specific needs, and a patient-centered approach can help ensure that they receive the best possible care and support.
Type Analysis
Sporadic ALS holds the largest market share in the type and segment of the Amyotrophic Lateral Sclerosis Treatment market in 2022, with 86.35 %.
Based on type, the market for Amyotrophic Lateral Sclerosis Treatment is segmented into Sporadic ALS and Familial ALS. This segment includes devices such as ventilators, feeding tubes, and mobility aids, which are used to support individuals with ALS as the disease progresses. These devices can help maintain respiratory function, prevent malnutrition, and can also improve the quality of life for individuals with ALS.
The ALS treatment market is highly competitive, with a variety of companies and research organizations working to develop new therapies and devices for the treatment of ALS. The market is driven by an increasing prevalence of the disease, advancements in medical technology and drug development, and a growing awareness of the need for effective treatments for ALS.
Drug Type Analysis
The Riluzole segment is estimated to be the most lucrative segment in the Global Amyotrophic Lateral Sclerosis market
By Drug Type, the market is further divided into Riluzole, Edaravone, and Nuedexta. However, there are several other drugs being developed and tested for the treatment of ALS, including Masitinib, CuATSM, and Arimoclomol, among others. The Riluzole segment has a market share of 37% and a projected CAGR of 5.6%, in 2022. While these drugs are still in the early stages of development and testing, they hold promise for the future of ALS treatment and may ultimately challenge the dominance of Riluzole and Edaravone in the market.
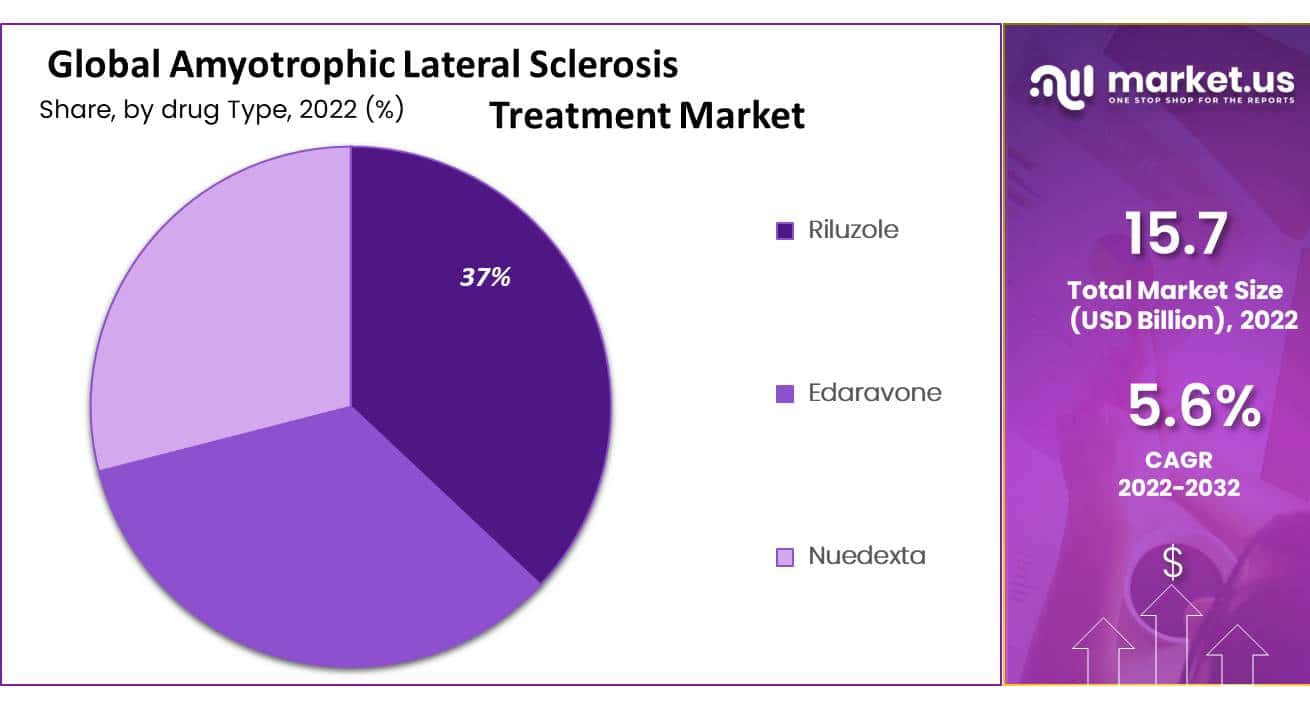
Treatment Analysis
The medication segment is estimated to be the most lucrative segment in the global Amyotrophic Lateral Sclerosis market
Based on Treatment Analysis, the market is segmented into Medication, Respiratory Therapy, Speech Therapy, Physical Therapy, Chemotherapy, and Stem Cell Therapy. The medication holds the largest revenue share of 22% and a projected CAGR of 5.59% during the forecast period. This refers to the use of drugs to treat or manage a particular disease or condition. Medications may be prescribed by a healthcare professional and can be administered orally, topically, intravenously, or via other routes.
Respiratory therapy, this treatment involves the use of various techniques and devices to improve respiratory function in individuals with breathing difficulties or lung diseases. Speech therapy, this type of therapy is designed to help individuals improve their speech and communication skills. Physical therapy, this treatment involves the use of physical techniques and exercises to help individuals recover from injuries or disabilities.
End-User Analysis
The hospital’s segment dominated
Based on end-user, the market is segmented into hospitals, specialty centers, Research and Academic Institutes, and Diagnostic Centers. Among these end-users, the hospital’s segment is estimated to be the most lucrative segment in the global Amyotrophic Lateral Sclerosis market, with the largest revenue share of 26% and a projected CAGR of 5.59% during the forecast period. These facilities provide diagnosis, treatment, and management of ALS patients, often in collaboration with specialized ALS centers.
ALS centers are specialized facilities that focus on the diagnosis and treatment of ALS patients. They provide a multidisciplinary approach to care, with teams of specialists including neurologists, respiratory therapists, physical and occupational therapists, and social workers. Research and academic institutions play a critical role in the ALS market by conducting clinical trials and research studies to develop new therapies and treatments for ALS patients.
Key Market Segments
Based on Type
- Sporadic ALS (SALS)
- Familial ALS (FALS)
Based on Drug Type
- Riluzole
- Edaravone
- Nuedexta
Based on Treatment Type
- Medication
- Respiratory Therapy
- Speech Therapy
- Physical Therapy
- Chemotherapy
- Stem Cell Therapy
- Other Treatments
Based on End-User
- Hospitals
- Specialty Centres
- Research and Academic Institutes
- Diagnostic Centers
- Other End Users.
Regional Analysis
North America Generated Highest Revenue Globally and Dominated the Global Amyotrophic Lateral Sclerosis Market
The global Amyotrophic Lateral Sclerosis market is growing worldwide, in 2022. North America held a 31% revenue share, and Europe is the second-largest market for ALS, driven by the high prevalence of ALS in some European countries and the presence of a well-established healthcare infrastructure. The Asia Pacific region is expected to experience significant growth in the ALS market due to the increasing awareness of ALS and improving healthcare infrastructure.
The Middle East and Africa and Latin America currently account for a relatively small share of the global ALS market. However, the increasing awareness of ALS and the growing focus on improving healthcare infrastructure in these regions could also potentially lead to new opportunities for market growth.
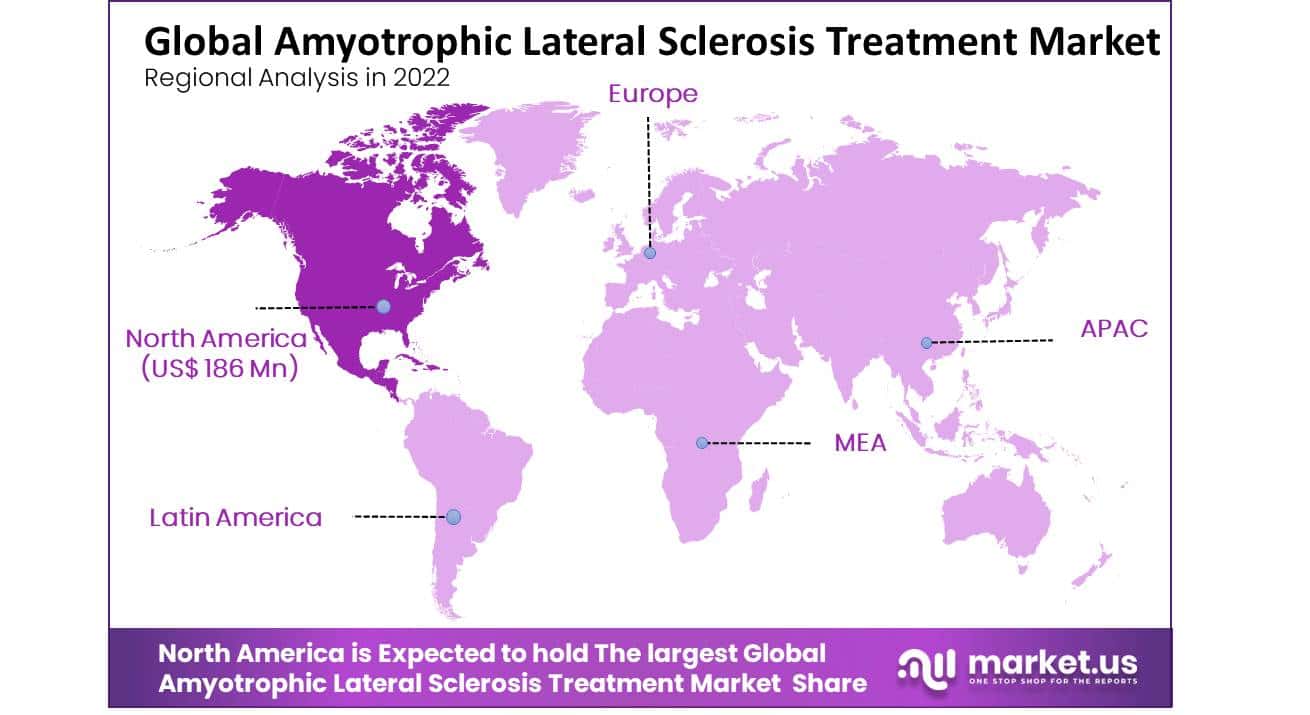
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global Amyotrophic Lateral Sclerosis market is a highly competitive market with several key players operating in the market. The market is dominated by a few major players who hold a significant market share. Some of the key players in the global ALS market include Mitsubishi Tanabe Pharma Corporation, Biogen Inc., Sanofi S.A., Italfarmaco S.p.A., and BrainStorm Cell Therapeutics Inc. Sanofi is the global pharmaceutical company that is involved in the development of treatments for ALS. Its product Rilutek (riluzole) is approved for the treatment of ALS in several countries. Other notable players in the global ALS market include Genervon Biopharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., and Cytokinetics, Inc.
Overall, the global ALS market is highly competitive, with several key players operating in the market. The market is driven by the increasing focus on research and development activities for developing novel treatments and therapies for ALS, along with the growing patient awareness of the disease and increasing healthcare infrastructure in emerging markets.
Market Key Players
- Sanofi
- Mitsubishi Tanabe Pharma Corporation
- BrainStorm Cell Limited
- Mylan N.V.
- Ionis Pharmaceuticals
- Biogen
- Covis Pharma
- Apotex Inc.
- Ascend Laboratories LLC
- ITF Pharma
- Genervon Biopharmaceuticals, LLC
- Bausch Health Companies Inc.
- ORPHAZYME A/S
- Orion Pharma Ltd.
- KRINGLE PHARMA, INC.
- Amylyx Pharmaceuticals Inc
- Sun Pharmaceutical Industries Ltd.
- Advanz Pharmaceutical
- Otsuka Pharmaceutical Co., Ltd.
- Other Key Players
Recent Developments
- In August 2021, the European Medicines Agency (EMA) granted orphan drug designation to Biogen’s tofersen for the treatment of ALS. Tofersen is an investigational therapy designed to target the genetic cause of ALS in patients with a specific gene mutation.
- In May 2021, the US Food and Drug Administration (FDA) approved the use of the neurostimulator device NeuroSphere for the treatment of ALS. The device is designed to stimulate nerves in the spinal cord to improve muscle control in patients with ALS.
- In March 2021, the ALS Association announced a collaboration with Biogen to launch the HEALEY ALS Platform Trial, which aims to accelerate the development of effective treatments for ALS. The trial will test multiple therapies simultaneously, with the goal of identifying promising treatments more quickly.
Report Scope
Report Features Description Market Value (2022) USD 600 Million Forecast Revenue (2032) USD 1021 Million CAGR (2023-2032) 5.6% Base Year for Estimation 2022 Historic Period 2016-2021 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type – Sporadic ALS, Familial ALS; By Drug Type – Riluzole, Edaravone, and Nuedexta; By Treatment -Medication, Respiratory Therapy, Speech Therapy, Physical Therapy, Chemotherapy, Stem Cell Therapy; By End Users- Hospitals, Specialty Centres, Research and Academic Institutes, Diagnostic Centers, Other End Users.
Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Sanofi, Mitsubishi Tanabe Pharma Corporation, BrainStorm Cell, Limited, Mylan N.V., Ionis Pharmaceuticals, Biogen, Covis Pharma Apotex Inc., Ascend Laboratories LLC, ITF Pharma, Genervon Biopharmaceuticals, LLC., Bausch Health Companies Inc., ORPHAZYME A/S, Orion Pharma Ltd., KRINGLE PHARMA, INC., Amylyx Pharmaceuticals Inc, Sun Pharmaceutical Industries Ltd., Advanz Pharmaceutical, Otsuka Pharmaceutical Co., Ltd., Other Key Players
Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is current size of Amyotrophic Lateral Sclerosis Treatment Market?In 2022, the global Amyotrophic Lateral Sclerosis Treatment Market was valued at US$ 600 million.
Who are major Key players of global Amyotrophic Lateral Sclerosis Treatment Market ?Sanofi, Mitsubishi Tanabe Pharma Corporation, BrainStorm Cell Limited, Mylan N.V., Ionis Pharmaceuticals, Biogen, Covis Pharma, Apotex Inc., Ascend Laboratories LLC, ITF Pharma, Genervon Biopharmaceuticals, LLC, Bausch Health Companies Inc., ORPHAZYME A/S, Other Key Players
What is projected Growth rate for global Amyotrophic Lateral Sclerosis Treatment Market ?Between 2023 and 2032, this market is estimated to register a CAGR of 5.6%.
 Amyotrophic Lateral Sclerosis (ALS) Treatment MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Amyotrophic Lateral Sclerosis (ALS) Treatment MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Sanofi
- Mitsubishi Tanabe Pharma Corporation
- BrainStorm Cell Limited
- Mylan N.V.
- Ionis Pharmaceuticals
- Biogen
- Covis Pharma
- Apotex Inc.
- Ascend Laboratories LLC
- ITF Pharma
- Genervon Biopharmaceuticals, LLC
- Bausch Health Companies Inc.
- ORPHAZYME A/S
- Orion Pharma Ltd.
- KRINGLE PHARMA, INC.
- Amylyx Pharmaceuticals Inc
- Sun Pharmaceutical Industries Ltd.
- Advanz Pharmaceutical
- Otsuka Pharmaceutical Co., Ltd.
- Other Key Players










