Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market By Product Type (Prolastin C, Aralast NP, Glassia, and Zemaira/Reespreza), By End-User (Hospitals, Specialty Clinics, and Pharmacies), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 95351
- Number of Pages: 370
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
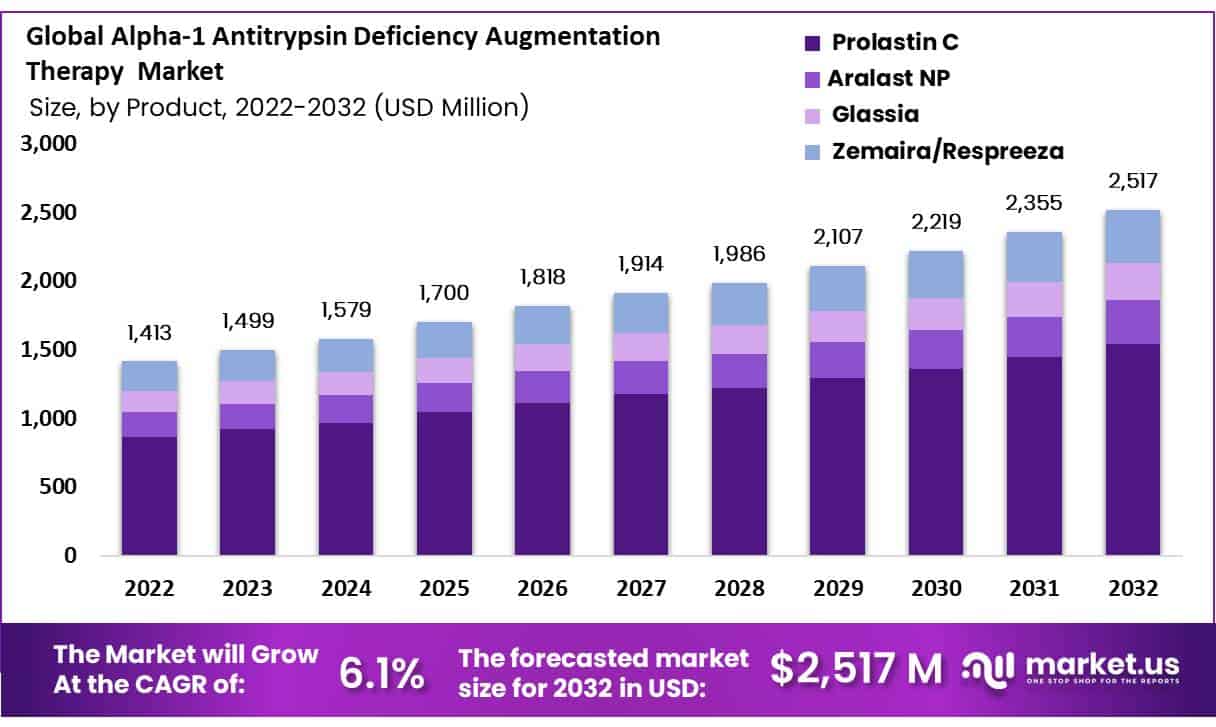
The global alpha-1 antitrypsin deficiency augmentation therapy market size is expected to be worth around USD 2,517 Mn by 2032 from USD 1,413 Mn in 2022, growing at a CAGR of 6.1% during the forecast period from 2022 to 2032.
Recent modifications in the technology of diagnostic tools pre-owned for genetic diseases have led to revolutionary progress in the treatment and diagnosis of AATD (Alpha-1 Antitrypsin Deficiency). AATD patients need daily regimented care, for which augmentation therapy is the most preferred option.
Researchers and manufacturers are presently striving to search for effective ways to offer them more elasticity in their treatment procedures. For the betterment of the Alpha-1 Antitrypsin Deficiency Treatment (AATD) Scenario, several pharmaceutical players like Octopharma and Biocerna LLC are involved in conducting research on existing and new molecules.
Alpha-1 Antitrypsin deficiency is termed an inherited condition described as an unpredicted or low level of alpha-1 protein synthesized by the liver. It often remains undiagnosed and generally affects the liver and lungs that, which can result in pulmonary diseases.
Key Takeaways
- Market Growth: The global AATD Augmentation Therapy market is set to reach USD 2,517 million by 2032, with a 6.1% CAGR.
- Technology Advancements: Recent genetic disease diagnostic tools enhance AATD diagnosis and treatment.
- Product Dominance: Prolastin C and Glassia lead AATD therapy, Prolastin C commanding the largest market share.
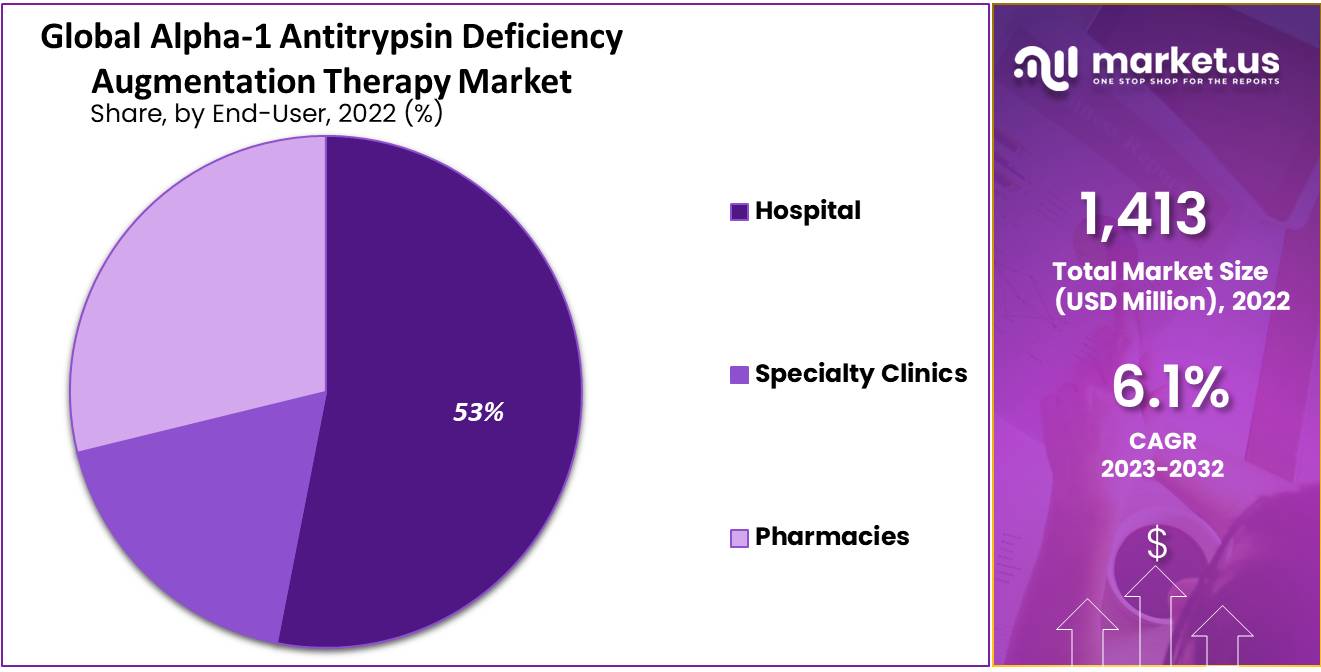
- End-User Segmentation: Hospitals drive AATD therapy demand due to genetic and respiratory disorders.
- Market Drivers: Genetic disorders, respiratory diseases, therapy effectiveness, and diagnostic advancements boost the market.
- Market Restraints: Augmentation therapy is not a cure, and improper AAT administration may cause vein damage.
- Market Opportunities: Using AAT from healthy donors and raising awareness, technology, and government support foster market growth.
- Trends: Over 80 AATD molecules in clinical trials and focus on market dynamics drive innovation.
- Regional Analysis: North America dominates due to awareness, government support, and diagnostics; Asia Pacific sees significant growth.
- Market Leadership: North America and Europe hold significant market share, with North America registered USD 579.5 million in 2022, due to disease prevalence and key companies.
Type Analysis
On the Basis of product type analysis, the segments of augmentation therapy include Prolastin C, Aralast NP, ,Glassia, Zemaira/Respreeza. Out of these, Prolastin C (manufactured by Grifols, S.A.) accounted for holding the highest market share during the forecast period. Hence it is considered to remain a dominant product in the upcoming days. As Europe regulatory authorities have given a green signal for Respreeza, it is projected to increase its growth with a high CAGR.
In adults with emphysema (lung disease) caused due to (AAT) Alpha-1 Antitrypsin Deficiency, Glassia (manufactured by Kamada Ltd.) is the most commonly used medicine. It is administered Intravenously at room temperature at a rate not exceeding 0.2mL/kg.
Aralast NP, synthesized from Human Alpha-1 Proteinase Inhibitor, is also used to treat emphysema in adults.
Hence, Prolastin C and Glassia are the most widely used treatment options in AATD Augmentation Therapy, which positively drives the growth of the Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market.
End-User Analysis
On the basis of end-user, the Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market is segmented into hospitals, specialty clinics, and pharmacies. Out of these, the hospital’s segment is accounted for holding the highest market share and a dominating segment of the market.
In developing economies, the hospital segment emerged as a public eye for the treatment of AATD Augmentation Therapy. It is attributable to the arrival of multispecialty care in different countries and the growing investment of private and public players in the healthcare sector.
The major factors responsible for the growth of the hospital segment in the AATD augmentation therapy market are the rising numbers of genetic disorders in the population combined with the rising prevalence of AATD. Thus, it necessitates the advancement of diagnostic techniques in hospitals and is contributing to the growth of this segment.
Followed by hospitals, pharmacies need to ensure the availability of medicines used to treat AATD as the number of congenital diseases is rising. Therefore, it will lead the pharmacy segment to report higher revenue during the forecast period.
Key Market Segments
Based on Product Type
- Prolastin C
- Aralast NP
- Glassia
- Zemaira/Respreeza
Based on End-User
- Hospitals
- Specialty Clinics
- Pharmacies
Drivers
Increasing genetic disorders and respiratory diseases to drive the market growth.
Severe AATD causes most genetic disorders which need hospital treatment, and whenever it comes to the severity in treating the patients of AATD, there are treatment options such as bronchodilators, proteinase inhibitors, oxygen therapy, and corticosteroids, for this purpose augmentation therapy is the most preferred one and hence a major driving factor supporting the growth of the market.
Other key driving factors responsible for market growth are greater involvement of key players, improvement in diagnostic tools, and an increasing number of hospitals with modifications in the healthcare sector.
Augmentation Therapy for disease management largely contributes to market growth.
In addition to it, augmentation therapy is found effective in the decline of mortality rate in patients with AATD and offers health benefits such as prevention of lung destruction, the elevation of A1AT in plasma and lung interstitium, and correction of deficiency as well as well-proven safety and efficacy profile.
Market key drivers are also involved in the modification of augmentation therapy treatment to reduce the side effects, which highly contributes towards the market growth.
Restraints
Augmentation Therapy is Not a Permanent Cure
Augmentation therapy is also termed replacement therapy. Hence it can only slow down lung deterioration and not permanently cure the AAT deficiency. As well, the lung damage that has already occurred will not reverse every time.
IV administration of AAT, usually once/week for a lifetime, is generally administered into the bloodstream via Intravenous Catheter. Such repeated insertion of catheters and needles has the chance of damaging veins.
But it can be prevented by proper care under the supervision of a qualified and experienced medical practitioner.
Opportunity
Availability of Healthy Donors
Augmentation therapy involves the usage of Alpha-1 antitrypsin (AAT) Protein obtained from healthy donors to expand the amount of Alpha-1 Antitrypsin in patients (having emphysema and those having severe AATD) lungs.
Hence it makes augmentation therapy an expensive and profit-generating treatment option and generates a large number of opportunities for the market driver to take an entry.
Technological Advancements and Rising Awareness
Furthermore, growing awareness among individuals regarding augmentation therapy, new drug development, innovation of new diagnostic techniques, and involvement of private and public authorities, as well as rising government campaigns in different countries, are creating more opportunities for leading players to focus on the augmentation therapy market.
Trends
Rising investment in R & D
Increasing investment in R&D, and capable pipeline products are anticipated to expand the growth of the AATD augmentation therapy market during the forecast period. Moreover, over 80 new molecules including inhalation therapy are being tested in clinical trial for alpha-1 antitrypsin deficiency.
Key players are focused on gaining knowledge about the detailed analysis of the competitive landscape and market dynamics regarding pricing analysis, recent and upcoming developments, the prevalence of AATD by major countries, and regulatory scenarios for major countries.
Regional Analysis
North America holds the largest market share
Based on regional analysis, the market is divided into major regions North America, Latin America Eastern Europe, Western Europe, Asia Pacific, and Middle East & Africa. The (AATD) Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market is dominated by North America.
Thus, North America holds the largest share among all regions in the market and will continue to dominate for the forecast period. This is achieved by the implementation of awareness programs considering the treatments and diagnosis of AATD in that particular region. Hence this will improve the market growth in the upcoming period.
The country segment domination is mainly the impact of factors and revised changes in regulations in the Market that affects the future and current ongoing trends of the Market.
Owing to the government’s participation in the control and prevention of genetic disorders and improvement in the diagnostic procedures for critical diseases, the Market of Asia Pacific is predicted to expand with a significant Compound Annual Growth Rate (CAGR) during the forecast period of 2023-2032. The rising population is the main factor responsible in Asia Pacific for market growth
However, North America registered a maximum revenue of US$ 579.5 Mn in 2022 and surely is going to lead in upcoming years. The dominance of North America is formed due to the rising prevalence of disease in Canada and the United States and the strategic appearance of key companies in that region. In terms of revenue, along with Asia Pacific, Europe is also projected as the prominent region to hold a large market share.

Key Regions
- North America
- The US
- Canada
- Mexico
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Several market players are now focusing on understanding the detailed analysis regarding market growth. For these, they are involved in conducting studies and experiments related to augmentation therapy and also increased their concentration on conducting Research and Development activities.
Forming a competitive landscape majority of key players are getting involved in collaborations, modifications in technology, advancement in diagnostic techniques and treatment procedures, and new product launches. The rising prevalence of respiratory disorders and increasing investments by major players are further stimulating market growth.
In past years, S.A. Kamada Ltd, Grifols, Shire plc (Baxalta), Teva Pharmaceutical industries ltd., and CSL Limited have been the major key players and accounted for the highest revenue generation. Grifols, S.A. accounted for maximum shareholding due to the sale of its product Prolastic C.
Thus, it is expected that Grifols will continue as a leading key player during the forecast period. More new players are emerging as the government of different regions is involved in the expansion of AATD Augmentation Therapy in their particular regional Market. Significantly this will drive the AATD Augmentation Therapy Market towards growth.
According to the Market report, below are the most prominent key players in the Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market.
Market Key Players
- Grifols, S.A. (Spain)
- Kamada Pharmaceuticals
- CSL Limited
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries ltd.
- GlaxoSmithKline PLC
- Arrowhead Pharmaceutical, Inc.
- LFB Biomedicaments S.A.
- Mereo BioPharma Group plc
- Intellia Therapeutics, Inc.
- Inhibrx, Inc.
- Centessa Pharmaceuticals (Z factor)
- Krystal Biotech
- Beam Therapeutics
- LOGICBIO THERAPEUTICS, INC.
- Apic Bio
- Pfizer Inc.
- Other Key Players
Recent Developments
- In November 2018 Grifols, S.A. launched Alfacare a therapy program to provide training and counseling to patients diagnosed with Alpha-1 Antitrypsin Deficiency. It helped patients with AATD to manage their disease properly by encouraging new habits.
- In June 2016, Kamada Pharmaceuticals received approval to glass a drug for AATD for self-infusion, enabling patients to self-administer a drug.
Report Scope
Report Features Description Market Value (2022) USD 1,413 Mn Forecast Revenue (2032) USD 2,517 Mn CAGR (2023-2032) 6.1% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type – Prolastin C, Aralast NP, Glassia, and Zemaira/Reespreza; and End User – Hospitals, Specialty Clinics, and Pharmacies. Regional Analysis North America – The US, Canada, & Mexico; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA. Competitive Landscape S.A. Kamada Ltd, Grifols, Shire plc (Baxalta), Teva Pharmaceutical industries ltd., and CSL Limited, Glaxosmithkline Plc, Takeda pharmaceuticals Industry Limited, Arrowhead Pharmaceutical, Inc., LFB Biomedicaments S.A., Mereo BioPharma Group plc, Intellia Therapeutics, Inc., Inhibrx, Inc., Centessa Pharmaceuticals (Z factor), Krystal Biotech, Beam Therapeutics, LOGICBIO THERAPEUTICS, INC., Apic Bio, Pfizer Inc., and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
Who are the prominent players in the global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market?Grifols, S.A. (Spain) Kamada Pharmaceuticals CSL Limited Takeda Pharmaceutical Company Limited Teva Pharmaceutical Industries ltd. GlaxoSmithKline PLC Arrowhead Pharmaceutical, Inc. LFB Biomedicaments S.A. Mereo BioPharma Group plc Intellia Therapeutics, Inc. Inhibrx, Inc. Centessa Pharmaceuticals (Z factor) Krystal Biotech Beam Therapeutics LOGICBIO THERAPEUTICS, INC. Apic Bio Pfizer Inc. Other Key Players
How big was the global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market in 2023?It was valued at USD 2 Billion in 2023
What is the growth rate of the global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market?The global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market is projected to grow at a CAGR of 6.10% between 2023 and 2032.
 Alpha-1 Antitrypsin Deficiency Augmentation Therapy MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Alpha-1 Antitrypsin Deficiency Augmentation Therapy MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Grifols, S.A. (Spain)
- Kamada Pharmaceuticals
- CSL Limited
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries ltd.
- GlaxoSmithKline PLC
- Arrowhead Pharmaceutical, Inc.
- LFB Biomedicaments S.A.
- Mereo BioPharma Group plc
- Intellia Therapeutics, Inc.
- Inhibrx, Inc.
- Centessa Pharmaceuticals (Z factor)
- Krystal Biotech
- Beam Therapeutics
- LOGICBIO THERAPEUTICS, INC.
- Apic Bio
- Pfizer Inc.
- Other Key Players










