Global Addison Disease Testing Market By Test Type (Laboratory testing, Imaging studies, Other specimen-based tests), By Technology (Immunoassays, Mass spectrometry, Point-of-care), By End-User (Hospitals, Diagnostic laboratories, and Clinics/ambulatory care settings), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Nov 2025
- Report ID: 167764
- Number of Pages: 346
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
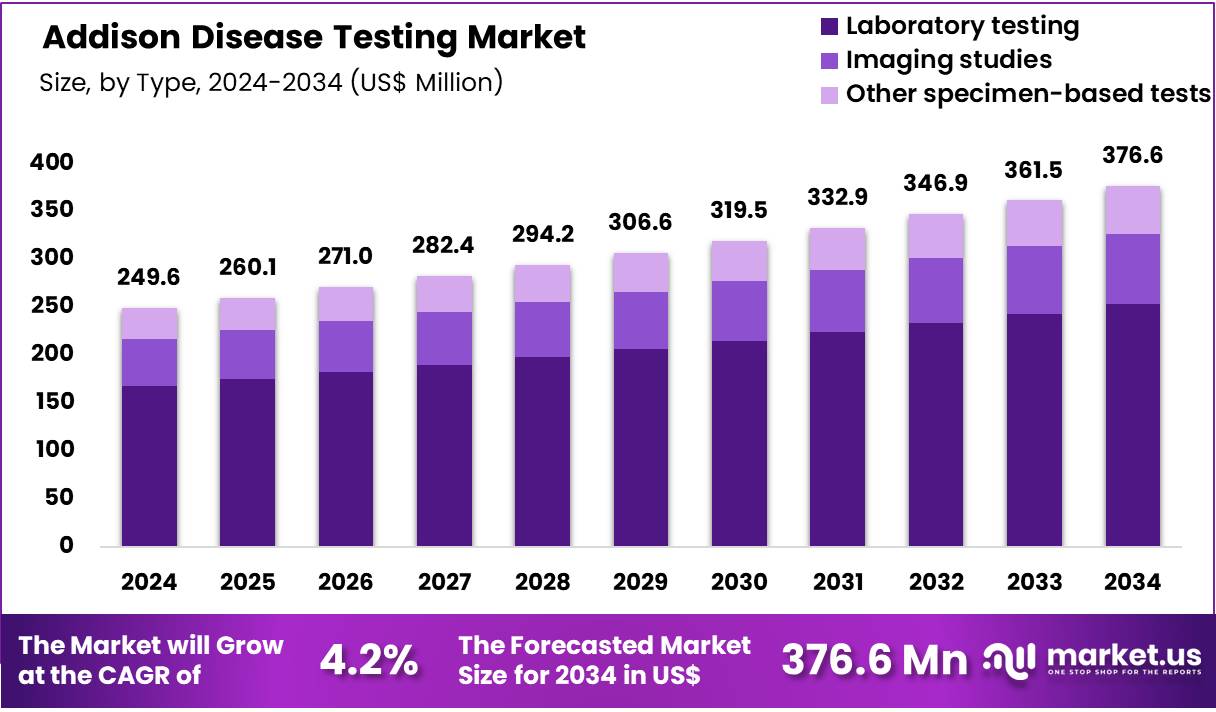
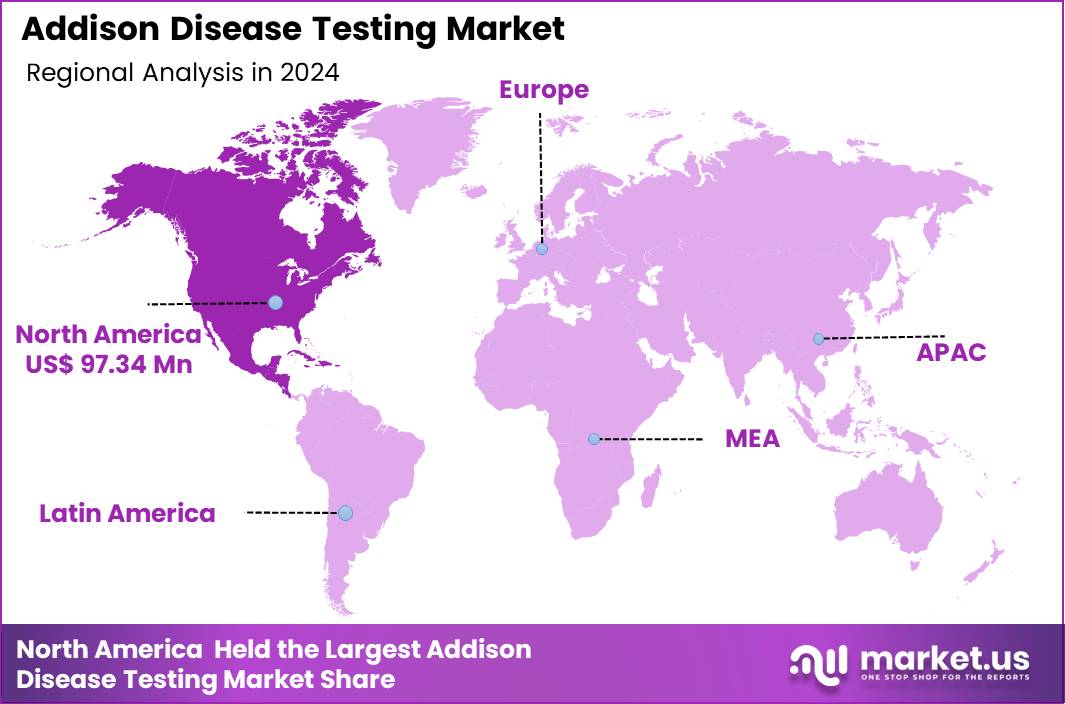
The Global Addison Disease Testing Market size is expected to be worth around US$ 376.6 Million by 2034 from US$ 249.6 Million in 2024, growing at a CAGR of 4.2% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.0% share with a revenue of US$ 97.34 Million.
The Addison Disease Testing Market is expanding as greater attention is placed on early adrenal insufficiency detection, driven by growing clinical awareness and the rising global burden of autoimmune endocrine disorders.
Addison disease testing typically involves biochemical laboratory evaluation, stimulation assays, immunoassay-based hormone quantification, imaging for adrenal morphology, and auxiliary specimen-based assessments used to confirm chronic adrenal cortex dysfunction. As the condition often presents with nonspecific symptoms, the need for accurate, sensitive, and timely testing has become central to improving diagnostic timelines.
According to NCBI, Addison’s disease is a rare condition with an annual incidence of about 0.6 to 0.8 cases per 100,000 people and a prevalence of 4 to 11 per 100,000. It typically affects women between the ages of 30 and 50, and while it can occur in all age groups, its symptoms may worsen under stress or infection. The prevalence of Addison disease is 40-60 cases per 1 million populations.
Modern testing approaches integrate cortisol and ACTH measurement, renin–aldosterone profiling, and adrenal antibody assessments processed in specialized endocrine laboratories. These tests support both initial diagnosis and long-term disease monitoring. Imaging modalities such as CT and MRI further assist clinicians in distinguishing autoimmune adrenalitis from infectious, hemorrhagic, or metastatic etiologies. Increasing emphasis on early symptom identification, emergency management, and adherence to international endocrine guidelines continues to support market growth.
With clinical demand rising and testing platforms modernizing, Addison disease diagnostics now form a critical component of endocrine practice. Ongoing technological improvements, tele-endocrinology expansion, and increased physician education efforts collectively strengthen this market’s outlook.
Key Takeaways
- In 2024, the market generated a revenue of US$ 249.6 Million, with a CAGR of 4.2%, and is expected to reach US$ 376.6 Million by the year 2034.
- The Test Type segment is divided into Laboratory testing, Imaging studies, and Other specimen-based tests, with Laboratory testing taking the lead in 2024 with a market share of 67.3%.
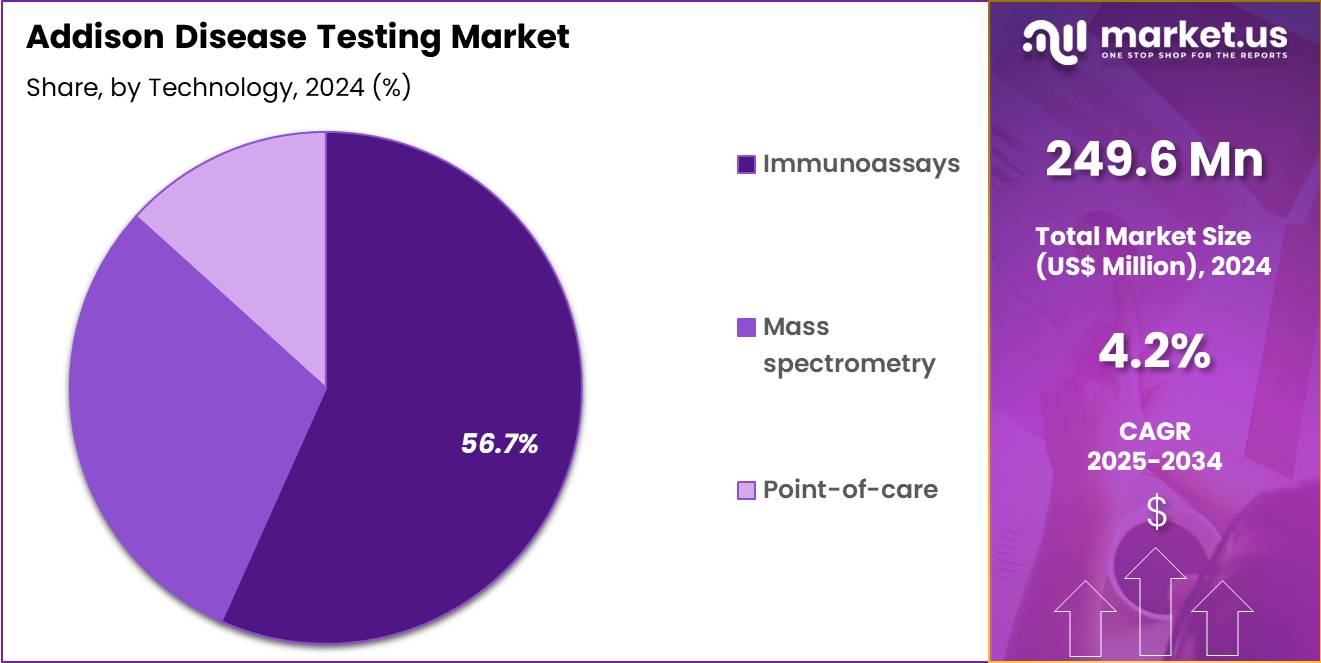
- The Technology segment is divided into Immunoassays, Mass spectrometry, and Point-of-care, with Immunoassays taking the lead in 2024 with a market share of 56.7%
- The End-User segment is divided into Hospitals, Diagnostic laboratories, and Clinics/ambulatory care settings, with Hospitals taking the lead in 2024 with a market share of 48.5%
- North America led the market by securing a market share of 39.0% in 2024.
Test Type Analysis
Laboratory testing holds the largest share of 67.3% in the Addison disease testing landscape because it remains the clinical gold standard for establishing primary adrenal insufficiency. The combination of baseline cortisol measurement, ACTH stimulation tests, plasma renin activity, and aldosterone levels forms the core diagnostic pathway.
These tests are routinely performed in specialized endocrine laboratories using strict quality-control protocols, allowing clinicians to differentiate between primary, secondary, and tertiary adrenal insufficiency. The central role of biochemical assays in confirming the disease ensures continued dominance of this category.
Imaging studies represent the next key segment, primarily used when laboratory results indicate adrenal insufficiency but the underlying cause requires further evaluation. CT and MRI scans of the adrenal glands help identify autoimmune destruction, hemorrhage, infection, or metastatic involvement. Imaging supports timely clinical decisions, particularly in emergency or atypical cases. Widespread access to cross-sectional imaging in hospitals sustains consistent demand.
Other specimen-based tests, including autoantibody measurements, urinary cortisol, or salivary cortisol assessments, support complementary diagnosis and long-term monitoring. Autoantibodies against 21-hydroxylase play an essential role in identifying autoimmune origins. These tests are increasingly adopted, especially in specialized endocrine centers, contributing to segment growth.
Technology Analysis
Immunoassays lead the technology segment as cortisol with 56.7% market share, ACTH, aldosterone, renin, adrenal antibodies, and related biomarkers are primarily quantified using automated chemiluminescent or radioimmunoassay platforms. These systems offer high sensitivity, rapid processing, and broad availability in hospital and diagnostic laboratory settings. Their reliability and widespread use in endocrine diagnostics maintain this segment’s leading position.
Mass spectrometry is gaining traction, especially for high-precision steroid profiling where assay interference is a concern. Liquid chromatography–mass spectrometry (LC-MS/MS) provides superior specificity for cortisol and aldosterone measurement, reducing cross-reactivity and improving diagnostic accuracy. Its adoption is increasing among reference laboratories and large tertiary centers.
Point-of-care testing is an emerging segment, primarily explored for rapid cortisol assessment in emergency settings. While still developing, these platforms are expected to expand as acute adrenal crisis management becomes a global priority.
End-User Analysis
Hospitals dominated the end-user category with 48.5% market share due to the clinical nature of Addison disease, which frequently requires emergency stabilization, dynamic testing, and imaging interventions. Most ACTH stimulation tests and adrenal imaging procedures are performed within hospital settings, reinforcing their leading share.
Diagnostic laboratories form the second-largest segment, supporting routine hormonal profiling, autoantibody detection, and confirmatory testing. Their role is strengthened by increasing referrals from primary care and endocrinology practices.
Clinics and ambulatory care settings utilize Addison disease testing for initial screening, symptom evaluation, chronic disease management, and long-term patient monitoring. Adoption continues to rise as outpatient endocrinology consultation volumes increase globally.
Key Market Segments
By Test Type
- Laboratory testing
- Imaging studies
- Other specimen-based tests
By Technology
- Immunoassays
- Mass spectrometry
- Point-of-care
By End-User
- Hospitals
- Diagnostic laboratories
- Clinics/ambulatory care settings
Drivers
Rising clinical awareness of adrenal insufficiency and earlier screening patterns
Growing clinical awareness of adrenal insufficiency is strengthening testing demand as physicians increasingly recognize early symptoms such as chronic fatigue, salt craving, weight loss, and hypotension, which were previously misattributed to general lifestyle-related conditions. Medical societies and endocrine associations have intensified educational programs aimed at primary care physicians, leading to faster referrals for ACTH stimulation tests and baseline cortisol measurements.
For example, the Endocrine Society’s updated guidelines encourage clinicians to test morning cortisol levels in patients presenting with recurrent dizziness or unexplained pigmentation, enabling earlier detection. Public health campaigns focusing on autoimmune disorders have also played a central role in educating patients to seek evaluation when symptoms persist despite routine treatment.
In emergency departments, rising awareness has resulted in more timely assessment of suspected adrenal crises, where rapid cortisol testing and early hydrocortisone administration are critical. Countries with well-established endocrine networks, such as the US, UK, and Germany, now routinely include adrenal insufficiency in differential diagnosis pathways for chronic fatigue.
As diagnostic delays decrease, more individuals undergo confirmatory laboratory testing, imaging studies, and adrenal autoantibody assessments. This systematic shift from reactive to proactive screening continues to drive growth across the Addison disease testing ecosystem.
Restraints
Limited availability of advanced endocrine testing in low-resource regions
A major restraint in the Addison Disease Testing Market is the limited reach of advanced endocrine diagnostic infrastructure in low- and middle-income countries, where specialized hormone testing equipment is scarce. Essential diagnostic tools such as automated immunoassay analyzers or LC-MS/MS platforms are often concentrated in only a few urban hospitals, forcing rural clinicians to rely on partial or delayed testing.
For example, in several African and Southeast Asian countries, ACTH stimulation tests are unavailable outside tertiary centers, resulting in long travel distances and delayed diagnosis for symptomatic patients. The lack of trained endocrinologists further exacerbates the issue, as proper interpretation of cortisol, aldosterone, and renin results requires specialist expertise.
In resource-limited settings, stockouts of reagents and inconsistent laboratory maintenance also affect test reliability and turnaround time. Even when basic hormonal testing is available, adrenal antibody testing or advanced steroid profiling is rarely accessible, limiting the ability to determine autoimmune causes.
Imaging capacity also remains restricted, with CT or MRI scanners either unavailable or too costly for widespread use. These structural barriers contribute to underdiagnosis, increased risk of adrenal crisis, and significant geographic disparities. Without substantial investment in laboratory networks, this restraint will continue to hinder broader market penetration.
Opportunities
Integration of LC-MS/MS and advanced immunoassay platforms for improved steroid profiling
An important opportunity for the Addison Disease Testing Market lies in integrating highly sensitive LC-MS/MS platforms and next-generation immunoassays into routine adrenal hormone evaluation. LC-MS/MS provides unmatched analytical accuracy for cortisol, aldosterone, 17-hydroxyprogesterone, and other adrenal steroids, eliminating cross-reactivity issues common with traditional immunoassays. This precision is especially beneficial for diagnosing borderline cases or detecting subtle hormonal variations in early-stage adrenal insufficiency.
For example, leading laboratories in North America and Europe now use LC-MS/MS panels to differentiate primary from secondary adrenal insufficiency with far greater reliability. Advanced immunoassay platforms such as chemiluminescent systems deliver rapid, automated hormone measurements suitable for high-volume hospital settings.
Integrating both technologies enables a hybrid testing model: fast initial screening through immunoassays, followed by confirmatory mass-spectrometry for complex or ambiguous cases. The growing availability of compact LC-MS/MS instruments further supports adoption in regional diagnostic labs that previously lacked such capabilities.
Pharmaceutical and biotechnology companies developing endocrine therapeutics also rely on detailed steroid profiling for clinical trials, expanding the use of these platforms. As clinical guidelines increasingly emphasize diagnostic precision, laboratories that upgrade to integrated testing ecosystems are positioned to meet rising demand while improving patient outcomes.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic and geopolitical factors influence the Addison Disease Testing Market by shaping consumer spending, supply chain stability, and access to diagnostic materials. Periods of economic slowdown typically shift household spending toward essential healthcare, which benefits at-home testing because it offers a lower-cost alternative to clinical diagnostics.
However, inflation-driven increases in raw material and logistics costs can raise kit prices, affecting affordability in price-sensitive markets. Geopolitical tensions also impact the sourcing of assay components, microfluidic cartridges, reagents, and lateral-flow materials, which are often manufactured across multiple countries. Disruptions in global trade routes or restrictions on chemical exports may slow production timelines and limit inventory availability for online and retail channels.
Public health policy changes in response to geopolitical events further influence demand. For example, global energy and food supply uncertainties increase consumer awareness of immunity, fatigue, and nutritional well-being, contributing to higher self-monitoring behavior.
Shifts in labor markets, such as the rise of remote work, encourage more people to adopt home diagnostics rather than visiting clinics. At the same time, increased government scrutiny over cross-border data transfers and digital health privacy may affect how testing companies store and process user data.
Latest Trends
Increasing use of high-precision panels for adrenal steroid profiling
A key trend shaping the Addison Disease Testing Market is the rapid uptake of high-precision LC-MS/MS panels for comprehensive adrenal steroid profiling. Traditional immunoassays often suffer from cross-reactivity, especially in cortisol testing, where structurally similar steroids can distort results. LC-MS/MS overcomes this challenge by separating and quantifying individual steroid molecules with exceptional specificity, making it the preferred tool in advanced endocrine centers.
Many hospitals have adopted LC-MS/MS panels that simultaneously measure cortisol, cortisone, DHEA-S, aldosterone, and related metabolites, providing clinicians with a detailed hormonal signature that improves diagnostic clarity. For example, in complex cases where morning cortisol values are borderline, LC-MS/MS can detect subtle deficiencies missed by conventional methods, reducing false-negative diagnoses.
Research institutions also rely on these panels for studying adrenal autoimmunity, congenital adrenal disorders, and steroid metabolism—further accelerating their use. The trend aligns with global healthcare modernization efforts, where laboratories prioritize analytical precision and reproducibility.
As instrument costs decrease and automated sample-preparation systems become more common, LC-MS/MS adoption is expanding beyond tertiary hospitals into regional diagnostic networks. The trend strengthens the shift toward comprehensive, data-rich endocrine assessments that enhance both diagnosis and long-term disease management.
Regional Analysis
North America is leading the Addison Disease Testing Market
North America represents the largest regional share of 39.0% in the Addison Disease Testing Market due to its advanced endocrine diagnostic infrastructure, widespread clinical awareness, and strong integration of standardized testing protocols. The region benefits from extensive availability of laboratory-based hormone assays, ACTH stimulation testing, and high-resolution imaging systems across hospitals and diagnostic centers.
These capabilities enable clinicians to diagnose adrenal insufficiency at earlier stages, reducing the risk of adrenal crisis and improving patient outcomes. The presence of leading diagnostic laboratories strengthens the market, as major providers routinely offer comprehensive cortisol, ACTH, renin, aldosterone, and adrenal antibody testing with rapid turnaround times.
Additionally, strong collaboration between endocrinologists, academic institutions, and national medical organizations supports continuous education, increasing recognition of autoimmune adrenalitis and secondary adrenal insufficiency. For example, US clinical guidelines emphasize early screening for at-risk populations, including individuals with type 1 diabetes, autoimmune thyroid disease, or chronic steroid use. Insurance coverage for endocrine testing further enhances accessibility, allowing a broad patient base to receive timely diagnosis and monitoring.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is the fastest-growing region in the Addison Disease Testing Market, driven by rising healthcare modernization, improving diagnostic infrastructure, and increasing recognition of adrenal disorders. Rapid urbanization, growing chronic disease burden, and expanding access to specialist care have heightened demand for early adrenal insufficiency evaluation.
Countries such as China, Japan, South Korea, and India are investing heavily in endocrine diagnostics, including immunoassay automation, hormone testing panels, and cross-sectional imaging. The growing prevalence of infectious etiologies such as tuberculosis-associated adrenal damage further accelerates testing needs in several parts of the region.
As healthcare professionals receive more training in identifying adrenal disorders, referrals for morning cortisol testing, ACTH stimulation tests, and adrenal imaging are rising. Diagnostic laboratory chains in Asia Pacific are rapidly adopting LC-MS/MS platforms to enhance accuracy in steroid profiling, a trend influenced by increasing patient expectations and competitive private healthcare markets.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the market include Quest Diagnostics, Mayo Clinic Laboratories, ARUP Laboratories, Eurofins, DiaSorin, Siemens Healthineers, Abbott Diagnostics, Randox Laboratories, Roche Diagnostics, and Other key players.
Quest Diagnostics supports Addison disease testing through a broad menu of endocrine assays, including cortisol, ACTH, aldosterone, renin activity, and adrenal antibody tests. Its nationwide laboratories, rapid turnaround, and advanced immunoassay technology make it a key provider for clinicians evaluating suspected adrenal insufficiency.
Mayo Clinic Laboratories offers comprehensive Addison disease diagnostics, integrating LC-MS/MS steroid profiling with adrenal antibody panels and ACTH stimulation testing. Its subspecialty endocrine expertise and interpretive reporting help clinicians differentiate primary, secondary, and autoimmune adrenal insufficiency with high analytical accuracy.
ARUP Laboratories provides an extensive range of adrenal testing services, including cortisol, ACTH, renin-aldosterone ratios, 21-hydroxylase antibodies, and specialized mass-spectrometry panels. Its strong reputation in complex endocrine diagnostics supports precise evaluation of adrenal dysfunction and autoimmune Addison disease.
Top Key Players
- Labcorp
- Quest Diagnostics
- Mayo Clinic Laboratories
- ARUP Laboratories
- Eurofins
- DiaSorin
- Siemens Healthineers
- Abbott Diagnostics
- Randox Laboratories
- Roche Diagnostics
- Other key players
Recent Developments
- In September 2025, Quest Diagnostics and Epic announced a first-of-its-kind collaboration designed to streamline and improve laboratory testing experience for health systems, hospitals and independent providers in the U.S.
- In March 2025, ARUP Laboratories expanded its AI-augmented screening tool for parasite detection, illustrating its capability for assay development and diagnostic innovation in reference lab settings.
- In November 2023, Mayo Clinic Laboratories and Progentec Diagnostics collaborated to bring advanced biomarker testing services to patients with autoimmune diseases — Rochester, Minn. & Oklahoma City. The partnership aims to increase access for providers and patients across the U.S. and select global markets.
Report Scope
Report Features Description Market Value (2024) US$ 249.6 Million Forecast Revenue (2034) US$ 376.6 Million CAGR (2025-2034) 4.2% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Test Type (Laboratory testing, Imaging studies, Other specimen-based tests), By Technology (Immunoassays, Mass spectrometry, Point-of-care), By End-User (Hospitals, Diagnostic laboratories, and Clinics/ambulatory care settings) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Labcorp, Quest Diagnostics, Mayo Clinic Laboratories, ARUP Laboratories, Eurofins, DiaSorin, Siemens Healthineers, Abbott Diagnostics, Randox Laboratories, Roche Diagnostics, and Other key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Addison Disease Testing MarketPublished date: Nov 2025add_shopping_cartBuy Now get_appDownload Sample
Addison Disease Testing MarketPublished date: Nov 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Labcorp
- Quest Diagnostics
- Mayo Clinic Laboratories
- ARUP Laboratories
- Eurofins
- DiaSorin
- Siemens Healthineers
- Abbott Diagnostics
- Randox Laboratories
- Roche Diagnostics
- Other key players









