Quick Navigation
Overview
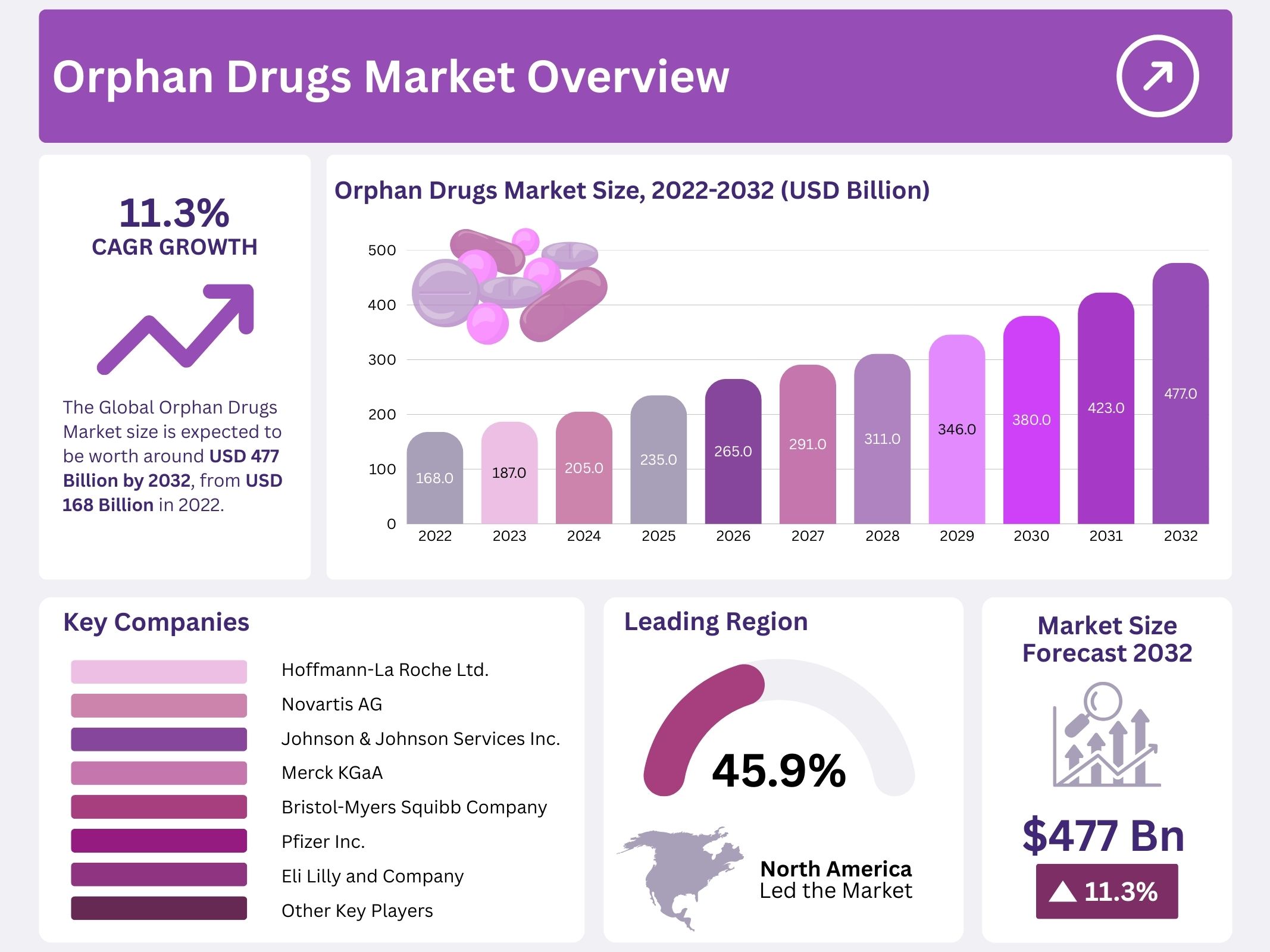
The Orphan Drugs Market is projected to grow from USD 168 billion in 2022 to nearly USD 477 billion by 2032. This reflects a compound annual growth rate (CAGR) of 11.3% during the forecast period. Growth is fueled by the large unmet medical need in rare diseases. Although each rare disease affects only a small population, globally millions of patients live with such conditions. For example, in the European Union, about 36 million people are affected. The World Health Assembly has also recognized rare diseases as a public health priority, reinforcing policy support for new therapies and diagnostics.
Clear regulatory incentives are encouraging sustained investment in the orphan drug sector. In the United States, the Orphan Drug Act provides tax credits, exemption from certain fees, and seven years of market exclusivity after approval. Similarly, the European Union grants ten years of market protection for designated orphan medicines. These frameworks help pharmaceutical companies recover high research and development costs, while reducing risks in small-population drug development. Transparent pathways, scientific guidance, and financial support from regulatory agencies further lower barriers, making it feasible for companies to bring novel treatments for rare diseases to market.
Recent approval trends highlight the strength of the pipeline. In 2024, the U.S. Food and Drug Administration (FDA) approved 50 novel drugs, with more than half targeting rare conditions. This indicates that companies are effectively using the orphan drug pathway to secure market entry. At the same time, major research institutions such as the U.S. National Institutes of Health report that only a fraction of the 10,000 known rare diseases have approved therapies. Healthcare costs for patients with rare diseases remain three to five times higher than average, creating payer interest and encouraging wider adoption of effective treatments.
Scientific progress is transforming feasibility in rare disease treatment. Advances in genetics, gene therapies, and data sharing accelerate time to diagnosis and improve study design for small patient groups. Research networks, such as the Rare Diseases Clinical Research Network coordinated by NIH, enable collaboration among study sites and patient groups, easing recruitment and evidence generation. At the global level, policy alignment is strengthening the ecosystem. The World Health Organization’s resolution on rare diseases and the European legislative framework provide predictable rules and long-term stability. Together, unmet need, regulatory support, rising approvals, and faster science create strong growth conditions for the orphan drug market.
Key Takeaways
- The global orphan drugs market is projected to reach USD 477 billion by 2032, registering a robust compound annual growth rate of 11.3%.
- Oncology therapy dominates the market revenue share, primarily due to increasing demand for advanced cancer treatment options addressing rare and complex oncological conditions.
- Biological orphan drugs lead the segment, favored for their proven effectiveness and relatively lower side-effect profile compared to conventional pharmaceutical alternatives.
- Hospital pharmacies hold the largest market share, supported by intravenous drug administration requirements, while online and retail pharmacies continue showing strong growth momentum.
- Market growth is primarily driven by rising rare disease prevalence, growing demand for immunomodulators, and supportive regulatory frameworks encouraging product development.
- High treatment costs, stringent regulatory frameworks, and limited disease awareness remain significant challenges restricting broader adoption of orphan drugs across regions.
- Opportunities arise from increasing rare disease diagnoses, coupled with FDA incentives, government funding, and supportive reimbursement schemes fostering research and commercialization.
- North America leads the global market due to favorable policies, while Europe and Asia-Pacific exhibit substantial growth potential driven by evolving healthcare ecosystems.
Regional Analysis
North America held a dominant market position, capturing more than a 45.9% share and holds US$ 77.2 Billion market value for the year. This dominance is supported by favorable legislation enabling rapid drug approvals and granting exclusivity status. A strong healthcare infrastructure and the presence of key industry players further contribute to market growth. In the United States, orphan drugs benefit from incentives such as tax credits, waived user fees, and seven years of marketing exclusivity after FDA approval. These factors collectively ensure steady expansion and strengthen the region’s leading market position.
Europe is projected to witness substantial growth in the orphan drugs market over the forecast period. The region’s large patient base and acceptance of advanced treatments for rare diseases are key drivers. Favorable regulatory frameworks and supportive reimbursement policies also stimulate adoption. Countries across Europe are increasingly investing in healthcare innovation and rare disease management. As a result, strong demand for innovative therapies is expected, creating profitable opportunities for pharmaceutical companies targeting rare disease treatment in the European market.
The Asia-Pacific region is anticipated to register the fastest growth in the global orphan drugs market. Rising healthcare spending, coupled with growing awareness of rare diseases, is fueling demand. Increasing prevalence of rare conditions is encouraging governments and organizations to promote access to treatments. Expansion of clinical trials in countries like China, India, and Japan is also accelerating growth. The region’s expanding healthcare infrastructure and patient outreach programs further support market expansion. Collectively, these factors position Asia-Pacific as a high-potential growth region for orphan drugs.
Latin America and the Middle East & Africa are expected to experience moderate but steady market expansion. Growth is supported by government initiatives aimed at improving community healthcare facilities and expanding oncology centers. In developing countries, greater emphasis on healthcare awareness and accessibility is increasing orphan drug availability. International collaborations and gradual improvements in healthcare policies are also contributing to regional progress. Although adoption is slower compared to developed markets, long-term opportunities are expected as awareness and investment levels continue to rise.
Segmentation Analysis
The oncology segment is projected to dominate the orphan drugs market due to the rising number of cancer cases globally. This therapy area continues to attract strong demand for advanced drugs that can treat or manage multiple cancer types. Growing awareness and research investments have strengthened its position in the market. With an increasing geriatric population requiring chronic care, oncology-based orphan drugs are expected to contribute significantly to revenue growth, thereby reinforcing their dominance across the forecast period in the global orphan drugs landscape.
Biologics are identified as the leading drug type in the orphan drugs market, holding the largest revenue share. The segment is anticipated to grow at a faster pace compared to non-biologicals. The rising approvals of biologic orphan drugs across various therapeutic applications have contributed to its dominance. Biologic therapies are increasingly used for rare disease management due to their effectiveness and reduced side effects, particularly in oncology. Their acceptance in developed nations has further fueled market expansion, while encouraging participation from both established players and new entrants.
Hospital pharmacies account for the largest distribution channel in the global orphan drugs market and are expected to maintain their dominance. The presence of skilled professionals and the need for intravenous administration of many orphan drugs drive this preference. Retail pharmacies are anticipated to grow at a moderate rate during the forecast period, supporting overall market expansion. Additionally, online pharmacies are gaining traction, supported by ease of access and consumer convenience, a trend that accelerated during the COVID-19 pandemic as patients increasingly purchased medications online.
The global orphan drugs market demonstrates growth opportunities driven by rising demand for effective therapies across critical disease areas. Oncology remains the key driver of market expansion, while biologics lead in drug type innovation. Distribution through hospital pharmacies ensures proper administration, while online platforms reflect evolving consumer trends. Collectively, these dynamics are anticipated to support sustained growth. Increasing research investments, regulatory support, and a growing elderly population are further expected to enhance adoption and market penetration of orphan drugs worldwide in the years ahead.
Key Players Analysis
The orphan drugs market is moderately competitive, with dominance concentrated among a small group of established pharmaceutical companies. These major players hold a significant market share due to their extensive resources, advanced R&D capabilities, and wide regulatory approvals. Their strategic advantage is further reinforced by strong brand recognition and established product pipelines. Despite their dominance, the increasing prevalence of rare diseases is opening opportunities for new entrants. This has resulted in a more dynamic competitive environment across the global orphan drugs market.
The competitive landscape is evolving as smaller players strengthen their position within the market. They are capturing a notable share, driven by rising cases of orphan diseases and growing demand for innovative treatments. These companies often focus on niche therapeutic areas with unmet needs. Their agility allows them to bring specialized products to market at a faster pace. This diversification of players is reshaping competition, challenging established firms, and fostering innovation in orphan drug development and commercialization.
In addition to market entry, strategic moves such as acquisitions, collaborations, partnerships, and mergers are shaping the industry structure. Leading companies are expanding their portfolios through product launches and licensing agreements to secure a stronghold in rare disease treatments. These efforts are designed to enhance geographic reach, strengthen clinical pipelines, and improve patient access to therapies. Such collaborations not only increase competitiveness but also accelerate the development of novel treatments, reinforcing growth opportunities in the global orphan drugs market while meeting rising patient needs worldwide.
Market Key Players
- Hoffmann-La Roche Ltd. (Switzerland)
- Novartis AG (Switzerland)
- Johnson & Johnson Services Inc. (U.S.)
- Merck KGaA (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Pfizer Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Novo Nordisk A/S (Denmark)
- Takeda Pharmaceutical Company Limited (Japan)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Kyowa Kirin Co., Ltd. (Japan)
- GlaxoSmithKline Plc
- AbbVie Inc.
- Sanofi S.A
- Amgen Inc. (U.S.)
- Biogen Inc. (U.S.)
- Celldex Therapeutics (U.S.)
- GSK plc. (U.K.)
- Eisai Co., Ltd. (Japan)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Alexion Pharmaceutical Inc.
- ALX Oncology Holdings Inc.
- BioMarin Pharmaceutical Inc.
- Agios Pharmaceuticals Inc.
- DAIICHI SANKYO Company Ltd.
- Other Key Players
Conclusion
The orphan drugs market is witnessing strong growth, driven by rising demand for rare disease treatments, favorable government incentives, and advancements in genetics and biologics. The sector benefits from regulatory support in major regions, helping companies overcome high research costs and enabling faster drug approvals. Oncology remains the leading therapy area, while biologics dominate drug types due to their effectiveness and safety. Hospital pharmacies continue to hold the largest distribution share, though online channels are expanding. Despite high treatment costs and limited awareness posing challenges, global collaborations, increasing diagnoses, and innovative therapies are creating significant opportunities for long-term expansion in the orphan drugs landscape.
Get in Touch with Us:
Market.us (Powered By Prudour Pvt. Ltd.)
Address: 420 Lexington Avenue, Suite 300, New York City, NY 10170, United States.
Contact No: +1 718 874 1545 (International), +91 78878 22626 (Asia).
Email: [email protected]
View More
Oncology Drugs Market || Neurology Market || Immunology Market || Gene Therapy Market || Cancer Gene Therapy Market || Cell and Gene Therapy Market










