Company Overview
Biogen Statistics: Biogen is a global biopharmaceutical company dedicated to discovering, developing, and delivering innovative therapies for individuals affected by serious and complex diseases. The company holds a broad and established portfolio of medicines for multiple sclerosis (MS), introduced the first approved therapy for spinal muscular atrophy (SMA), co-developed treatments addressing key Alzheimer’s disease pathologies, and launched the first approved therapy targeting a genetic cause of amyotrophic lateral sclerosis (ALS). Biogen’s research focus spans neurology, specialized immunology, and rare diseases, supported by a strong combination of in-house strategic partnerships, R&D programs, and targeted acquisitions.
Its marketed portfolio includes PLEGRIDY, VUMERITY, TECFIDERA, AVONEX, and TYSABRI for MS; SPINRAZA for SMA; SKYCLARYS aimed at Friedreich’s ataxia (FA); QALSODY for ALS; and FUMADERM for severe plaque psoriasis. The company operates through a single business segment, generating revenue from the discovery, development, and commercialization of advanced therapies for serious medical conditions. Biogen maintains a strong international footprint, operating in more than 80 countries across Latin America, Canada, Europe, the Middle East, and the Asia-Pacific region.
History of Biogen
1900’s
- 1978: Biogen was established in Geneva, Switzerland, by visionary scientists, including Nobel laureates Walter Gilbert and Phillip Sharp, aiming to leverage biotechnology for the treatment of serious human diseases.
- 1982: The company formed Biogen N.V. in the Netherlands and began developing its first line of recombinant DNA-based products.
- 1983: Biogen became a publicly traded company, marking its official entry into the global biotechnology sector.
- 1985: The company introduced its first licensed product, Intron, a recombinant interferon therapy designed for treating viral infections and certain cancers.
- 1996: Biogen launched Avonex, a breakthrough treatment for multiple sclerosis (MS), which went on to become one of the world’s most prescribed MS therapies.
2000’s
- 2003: Biogen merged with IDEC Pharmaceuticals, forming Biogen Idec and becoming one of the largest biotechnology firms globally.
- 2004: The company strengthened its MS portfolio with the introduction of Tysabri, further solidifying its leadership in neurological therapies.
- 2015: The company rebranded itself as Biogen, signaling a renewed commitment to neurology and neurodegenerative research.
- 2016: Biogen spun off its hemophilia division to create Bioverativ, enabling a sharper focus on neurology and rare diseases.
- 2017: The company achieved a major scientific milestone with Spinraza, the first FDA-approved treatment for spinal muscular atrophy (SMA).
- 2021: Aduhelm, an innovative therapy for Alzheimer’s disease, received FDA approval, marking Biogen’s expansion into Alzheimer’s care.
- 2023: Leqembi, developed in collaboration with Eisai, became the first Alzheimer’s drug approved by the FDA to slow disease progression.
- 2024: Biogen expanded its footprint in specialized immunology and rare diseases through strategic acquisitions and strengthened its R&D capabilities.
(Source: Company Website)
Employee Analysis
- As of December 31, 2024, Biogen Inc. employed about 7,605 people worldwide.
- Out of the total workforce, nearly 4,255 employees were based in the United States.
- Around 3,350 employees were stationed in international locations.
- Within the U.S., ethnic or racial minorities held approximately 9% of all manager-level and higher roles.
- On a global scale, women occupied nearly 3% of positions at the director level and above.
- These figures highlight Biogen’s commitment to workforce diversity and gender inclusion across its global operations.
(Source: Company Website)
Financial Analysis
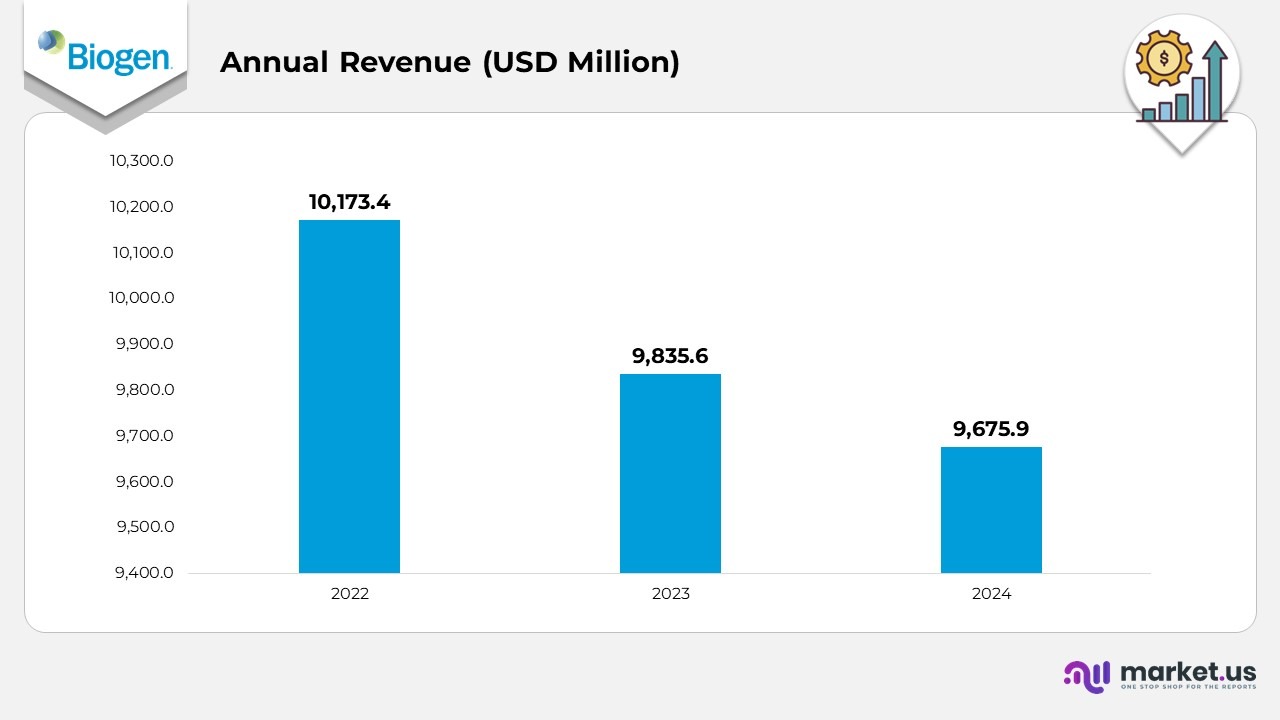
- In 2022, Biogen achieved an annual revenue of USD 10,173.4 million, marking one of its strongest financial performances, largely supported by its well-established multiple sclerosis and rare disease product lines.
- In 2023, annual revenue declined to USD 9,835.6 million, showing a 3% year-over-year decrease as competition in the multiple sclerosis market intensified and generic alternatives entered key regions.
- In 2024, Biogen’s revenue fell further to USD 9,675.9 million, a 6% decline from 2023, reflecting the company’s continued strategic transition toward rare diseases, biosimilars, and Alzheimer’s therapies to counteract weakening MS sales.
- Between 2022 and 2024, total revenue contracted by around 9%, highlighting Biogen’s gradual shift from its traditional product base toward newer therapeutic growth areas.
Product Revenue Analysis
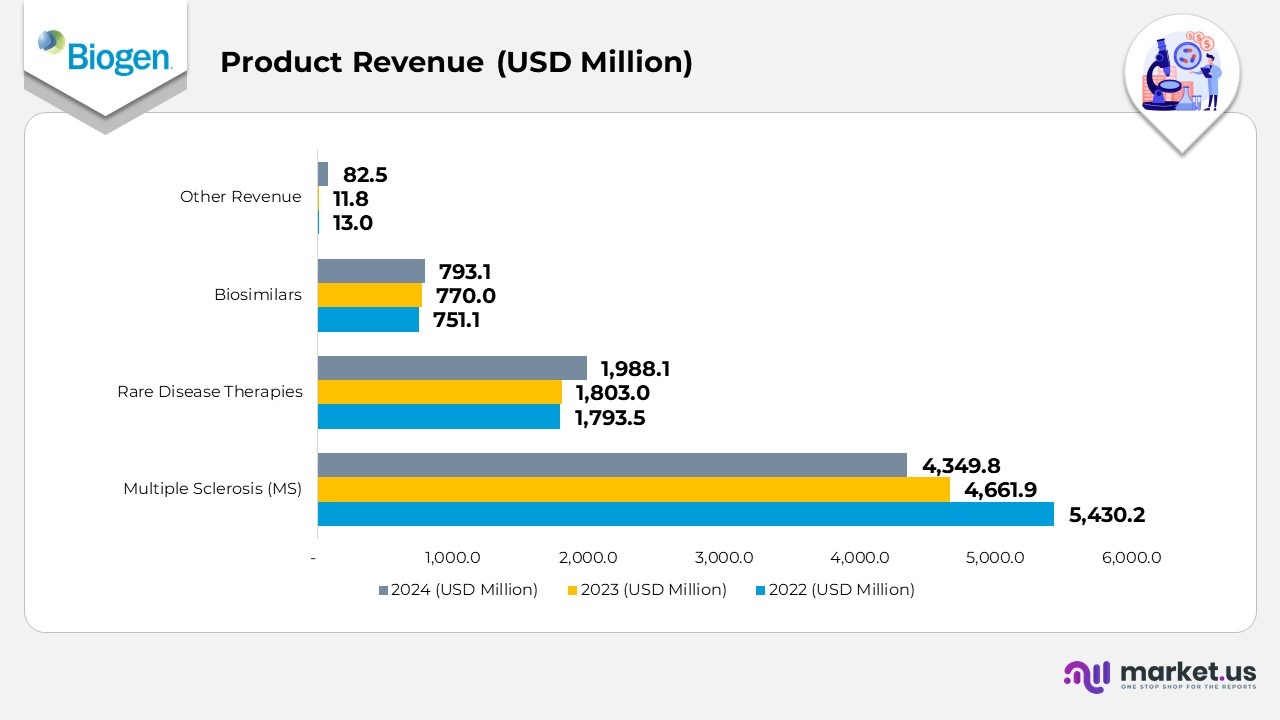
- In 2024, Biogen generated USD 4,349.8 million from its multiple sclerosis (MS) portfolio, down from USD 4,661.9 million in 2023 and USD 5,430.2 million in 2022, showing a steady decline due to competitive market pressures and changing treatment preferences.
- Rare disease therapies posted strong growth, contributing USD 1,988.1 million in 2024 versus USD 1,803.0 million in 2023 and USD 1,793.5 million in 2022, supported by the wider uptake of key products such as Spinraza and Qalsody.
- The biosimilars segment recorded revenue of USD 793.1 million in 2024, increasing from USD 770.0 million in 2023 and USD 751.1 million in 2022, driven by expanded market reach and successful new product introductions in Europe.
- The other revenue segment rose sharply to USD 82.5 million in 2024, compared to USD 11.8 million in 2023 and USD 13.0 million in 2022, primarily reflecting higher licensing income and strategic collaborations.
- Overall, 2024 represented a transition year for Biogen, where lower MS revenues were balanced by significant growth in rare diseases, biosimilars, and other income streams, emphasizing the company’s move toward a more diversified therapeutic portfolio.
Multiple Sclerosis (MS)
- Global TYSABRI revenue declined by USD 161.9 million, dropping from USD 1,876.9 million in 2023 to USD 1,715.0 million in 2024, representing a decrease of 6%, mainly due to intensified market competition and lower pricing across international markets.
- Global TECFIDERA revenue fell by USD 45.4 million, from USD 1,012.5 million in 2023 to USD 967.1 million in 2024, a decline of 5%, attributed to reduced demand following the entry of multiple generic versions in North America, Brazil, and several European Union countries.
- Global Interferon revenue decreased by USD 137.7 million, from USD 1,105.7 million in 2023 to USD 968.0 million in 2024, marking a 5% decline, primarily driven by lower demand as patients continue shifting toward higher efficacy treatments.
- Global VUMERITY revenue grew by USD 51.7 million, increasing from USD 576.3 million in 2023 to USD 628.0 million in 2024, reflecting a 0% rise supported by stronger global demand for the therapy.
Rare Disease
- S. SPINRAZA revenue rose by USD 15.2 million, increasing from USD 610.5 million in 2023 to USD 625.7 million in 2024, reflecting a 2.5% growth mainly driven by favorable net pricing, partially offset by a slight decline in overall demand.
- The rest of the world SPINRAZA revenue declined by USD 183.2 million, dropping from USD 1,130.7 million in 2023 to USD 947.5 million in 2024, a decrease of 2%, primarily due to the loss of an annual tender in Russia (impacting approximately USD 45.0 million) and additional effects from shipment timing and unfavorable currency exchange rates.
- Global SKYCLARYS revenue reached USD 382.5 million in 2024, comprising USD 301.1 million from U.S. sales recognized beginning in Q4 2023 after the Reata acquisition and USD 81.4 million from international markets following EU approval and commercial launch in early 2024.
- Global QALSODY revenue totaled USD 32.4 million in 2024, marking the product’s contribution to Biogen’s expanding rare disease portfolio.
Biosimilars
- For 2024 compared with 2023, the rise in biosimilar revenue was mainly attributed to higher sales volumes of BENEPALI, although this growth was partially offset by lower pricing driven by competitive market pressures.
(Source: Biogen Inc. SEC Filings)
Research and Development Expenditure
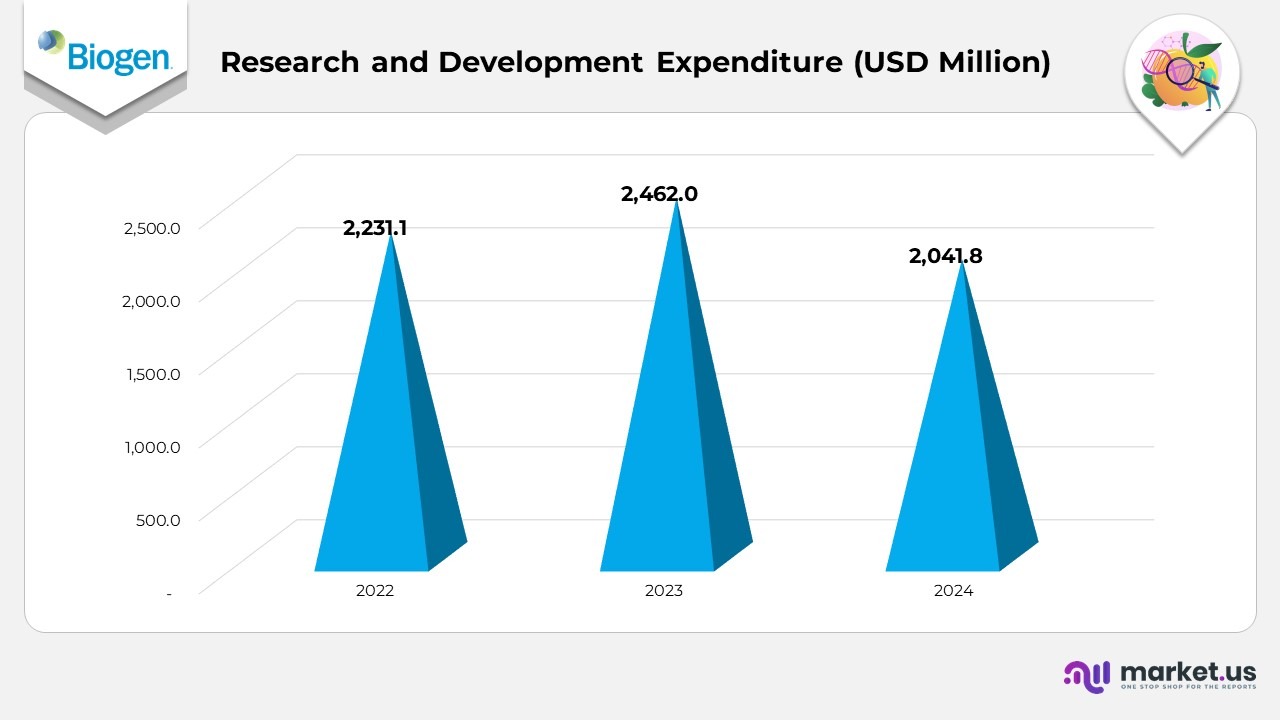
- Research and development (R&D) expenses accounted for 1% of total revenue in 2024, compared to 25.0% in 2023 and 21.9% in 2022, reflecting a notable year-over-year decline in 2024.
- The reduction in R&D spending for 2024 was primarily due to the absence of approximately USD 197.0 million in equity-based compensation costs recorded in 2023 following the Reata acquisition.
- Additional savings were achieved through cost-optimization measures implemented under Biogen’s portfolio prioritization initiatives and the Fit for Growth program, which streamlined operations and improved financial efficiency.
- The decline was further influenced by the completion of major clinical trial activities and related close-out costs that were incurred in 2023.
- These factors were partially offset by approximately USD 48.5 million in step-up amortization tied to SKYCLARYS inventory and about USD 42.5 million in equity-based compensation expenses associated with the HI-Bio acquisition recognized during 2024.
- Overall, the shift underscores Biogen’s disciplined R&D approach, focusing investments on high-potential therapeutic areas while managing portfolio and operational efficiency.
(Source: Biogen Inc. SEC Filings)
Global Developments and Regulatory Progress of LEQEMBI (Lecanemab)
United States
- In January 2025, the U.S. FDA approved LEQEMBI’s monthly intravenous (IV) maintenance dosing for patients with early Alzheimer’s disease, expanding its treatment flexibility.
- Also in January 2025, the FDA accepted the Biologics License Application (BLA) for LEQEMBI’s subcutaneous autoinjector, designed for weekly maintenance dosing, with a PDUFA action date set for August 31, 2025.
- In July 2024, Eisai presented new data from the CLARITY AD open-label extension study, showing that three years of continuous LEQEMBI therapy led to a clinically meaningful reduction in cognitive decline among early Alzheimer’s patients.
Rest of World
- In January 2025, Biogen and Eisai provided an update on the EU regulatory review for LEQEMBI, following the CHMP’s positive opinion in November 2024. The European Commission (EC) requested further assessment of newly available safety data and clarification on risk minimization language to be reviewed during the February 2025 CHMP meeting.
- In December 2024, Mexico’s Federal Commission for the Protection Against Sanitary Risk (COFEPRIS) granted marketing approval for LEQEMBI.
- In November 2024, Biogen and Eisai launched LEQEMBI in South Korea, after approval by the Ministry of Food and Drug Safety (MFDS) in May 2024.
- In October 2024, the Therapeutic Goods Administration (TGA) of Australia issued an initial decision not to register lecanemab, but Eisai submitted a reconsideration request in December 2024.
- In August 2024, LEQEMBI received dual approvals from the Medicines and Healthcare Products Regulatory Agency (MHRA) in Great Britain and the Ministry of Health and Prevention in the United Arab Emirates.
- In July 2024, LEQEMBI gained approvals in Hong Kong and Israel, expanding its reach across Asia and the Middle East.
- In June 2024, Biogen and Eisai launched LEQEMBI in China, following its January 2024 approval by the National Medical Products Administration (NMPA).
(Source: Biogen Inc. SEC Filings)
Biogen’s Patent Portfolio Across Major Therapeutic Areas
| Product | Territory | Patent No. | General Subject Matter |
|---|---|---|---|
| TECFIDERA | U.S. | 8399514 | Methods of treatment |
| Europe | 1131065 | Formulations of dialkyl fumarates and their use for treating autoimmune diseases | |
| PLEGRIDY | U.S. | 7446173 | Polymer conjugates of interferon beta-1a |
| U.S. | 8524660 | Methods of treatment | |
| U.S. | 8017733 | Polymer conjugates of interferon beta-1a | |
| Europe | 1656952 | Polymer conjugates of interferon-beta-1a and uses thereof | |
| Europe | 1476181 | Polymer conjugates of interferon-beta-1a and uses thereof | |
| TYSABRI | U.S. | 7807167 | Methods of treatment |
| U.S. | 9493567 | Methods of treatment | |
| U.S. | 10233245 | Methods of treatment | |
| Europe | 1485127 | Methods of use | |
| Europe | 2676967 | Methods of use | |
| FAMPYRA | Europe | 1732548 | Sustained-release aminopyridine compositions for increasing walking speed in patients with MS |
| Europe | 2377536 | Sustained-release aminopyridine compositions for treating MS | |
| VUMERITY | U.S. | 8669281 | Compounds and pharmaceutical compositions |
| U.S. | 9090558 | Methods of treatment | |
| U.S. | 10080733 | Crystalline forms, pharmaceutical compositions and methods of treatment | |
| Europe | 2970101 | Crystalline forms, pharmaceutical compositions and methods of treatment; prodrugs of fumarates and their use in treating various diseases | |
| SPINRAZA | U.S. | 7101993 | Oligonucleotides containing 2’-O-modified purines |
| U.S. | 7838657 | SMA treatment via targeting of SMN2 splice site inhibitory sequences | |
| U.S. | 8110560 | SMA treatment via targeting of SMN2 splice site inhibitory sequences | |
| U.S. | 8361977 | Compositions and methods for modulation of SMN2 splicing | |
| U.S. | 8980853 | Compositions and methods for modulation of SMN2 splicing | |
| U.S. | 9717750 | Compositions and methods for modulation of SMN2 splicing | |
| U.S. | 9926559 | Compositions and methods for modulation of SMN2 splicing | |
| U.S. | 10266822 | SMA treatment via targeting of SMN2 splice site inhibitory sequences | |
| U.S. | 10436802 | Methods for treating spinal muscular atrophy | |
| Europe | 1910395 | Compositions and methods for modulation of SMN2 splicing | |
| Europe | 2548560 | Compositions and methods for modulation of SMN2 splicing | |
| Europe | 3305302 | Compositions and methods for modulation of SMN2 splicing | |
| Europe | 3308788 | Compositions and methods for modulation of SMN2 splicing | |
| Europe | 3449926 | Compositions and methods for modulation of SMN2 splicing | |
| ADUHELM | U.S. | 8906367 | Method of providing disease-specific binding molecules and targets |
| U.S. | 10131708 | Methods of treating Alzheimer’s disease |
(Source: Company Website)
Recent Developments
- In October 2025, Biogen and Eisai Co., Ltd. announced that Health Canada granted a Notice of Compliance with Conditions (NOC/c) for LEQEMBI (lecanemab), a humanized anti-soluble combined amyloid-beta monoclonal antibody. The approval covers adult patients with mild cognitive impairment (MCI) or mild dementia due to Alzheimer’s disease (early AD) who are ApoE ε4 non-carriers or heterozygotes with confirmed amyloid pathology.
- In October 2025, Biogen entered an exclusive worldwide licensing agreement with Vanqua Bio, gaining rights to its preclinical oral C5aR1 antagonist. This acquisition strengthens Biogen’s immunology pipeline, advancing an immune-based therapeutic mechanism aimed at addressing multiple inflammatory disorders with significant unmet clinical needs.
- In October 2025, Biogen and Eisai Co., Ltd. announced the U.S. availability of LEQEMBI IQLIK™ (lecanemab-irmb), a subcutaneous maintenance dosing regimen for treating Alzheimer’s disease in patients with mild cognitive impairment or mild dementia (early AD).
- In September 2025, the Therapeutic Goods Administration (TGA) of Australia approved LEQEMBI (lecanemab) for adults with mild cognitive impairment or mild dementia due to Alzheimer’s disease. The approval applies to ApoE ε4 non-carriers or heterozygous carriers, marking a significant regulatory milestone in the Asia-Pacific region.
Moreover
- In September 2025, Biogen completed the acquisition of Alcyone Therapeutics, expanding its neuroscience delivery technology portfolio. The collaboration advances the ThecaFlex DRx system, an implantable subcutaneous port and catheter device developed for intrathecal delivery of antisense oligonucleotides (ASOs), enhancing treatment options for neurological conditions.
- In September 2025, the European Commission (EC) granted marketing authorization for ZURZUVAE (zuranolone), indicated for the treatment of postpartum depression (PPD) in adult women following childbirth, further broadening Biogen’s psychiatric and women’s health portfolio.
- In August 2025, the U.S. Food and Drug Administration (FDA) approved the Biologics License Application (BLA) for LEQEMBI IQLIK™, a once-weekly subcutaneous autoinjector containing 360 mg/1.8 mL (200 mg/mL), allowing administration in approximately 15 seconds. This approval enhances patient convenience for Alzheimer’s maintenance therapy.
- In August 2025, Biogen and Eisai Co., Ltd. launched LEQEMBI in Austria on August 25, 2025, with a Germany launch scheduled for September 1, 2025, following its European Commission approval in April 2025 as the first treatment addressing an underlying cause of Alzheimer’s disease.
- In July 2025, Biogen announced a USD 2 billion investment in its manufacturing operations in North Carolina’s Research Triangle Park (RTP). The initiative focuses on expanding antisense oligonucleotide (ASO) manufacturing, developing multi-platform fill-finish facilities, and modernizing production through advanced automation and artificial intelligence, reinforcing Biogen’s long-term manufacturing and innovation strategy.
(Source: Biogen Inc. Press Releases)