Global Structural Heart Devices Market- By Product (Heart Valve Devices, Annuloplasty Rings, Surgical Heart Valves, Occluders and Delivery Systems, Transcatheter Heart Valves, and Other Products), By Indication (Atrial Septal Defect, Patent Foramen Ovale, Ventricular Septal Defect, Aortic Valve Stenosis, and Other Indications), By Procedure (Replacement Procedures, Repair Procedures, TAVR Procedures, SAVR Procedures, Valvuloplasty, Annuloplasy, and TMVR Procedures), By End-User (Hospitals, Ambulatory Surgical Centers, and Other End-Users), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2024-2032
- Published date: Nov 2023
- Report ID: 101402
- Number of Pages: 232
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
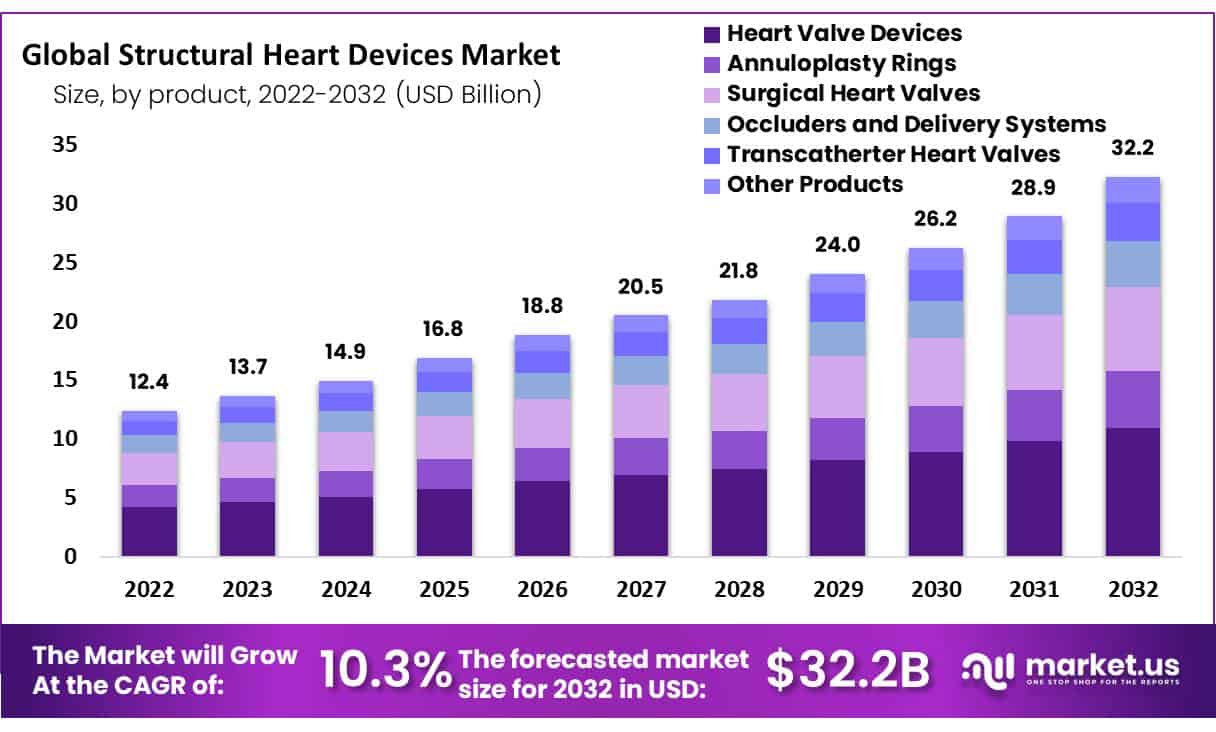
Global Structural Heart Devices Market size is expected to be worth around USD 32.2 Billion by 2032 from USD 13.7 Billion in 2023, growing at a CAGR of 10.3% during the forecast period from 2024 to 2032.
Structural heart diseases are an extensive percutaneous treatment for patients suffering from acquired heart disease and CHD, including functional and structural abnormalities of great valves, heart valves, and cardiac chambers. All these conditions may be witnessed by birth but can occur later in life due to the wear and tear from infection.
The high significance of target disease is the primary driver of the market. Around 60 million people in the US have structural abnormalities in their hearts. This accounts for almost 20-25% of the overall population. It indicates that there is a huge scope for structural heart devices owing to the increasing number of heart defects.
According to Children’s HeartLink, approximately 1% of infants are born with congenital heart disease (CHD), a condition caused by structural heart abnormalities. In countries with advanced pediatric cardiac care, most of these children grow into adulthood. However, about 90% of children with CHD globally are unable to access the necessary treatment due to a lack of availability or prohibitive costs.
To bridge this gap, Children’s HeartLink provides training for local pediatric heart teams in countries such as Bangladesh, Brazil, China, India, Malaysia, and Vietnam, improving the availability and affordability of care for vulnerable children.
Key Takeaways
- The structural heart devices market size is expected to grow from USD 13.7 billion in 2023 to USD 32.2 billion by 2032.
- The market will grow at a CAGR of 10.3% during the forecast period from 2024-2032.
- Around 60 million people in the US have structural abnormalities in their hearts.
- Structural heart defects affect 20-25% of the overall US population.
- The heart valve devices segment of the market is expected to hold a dominant share of 34% during the forecast period.
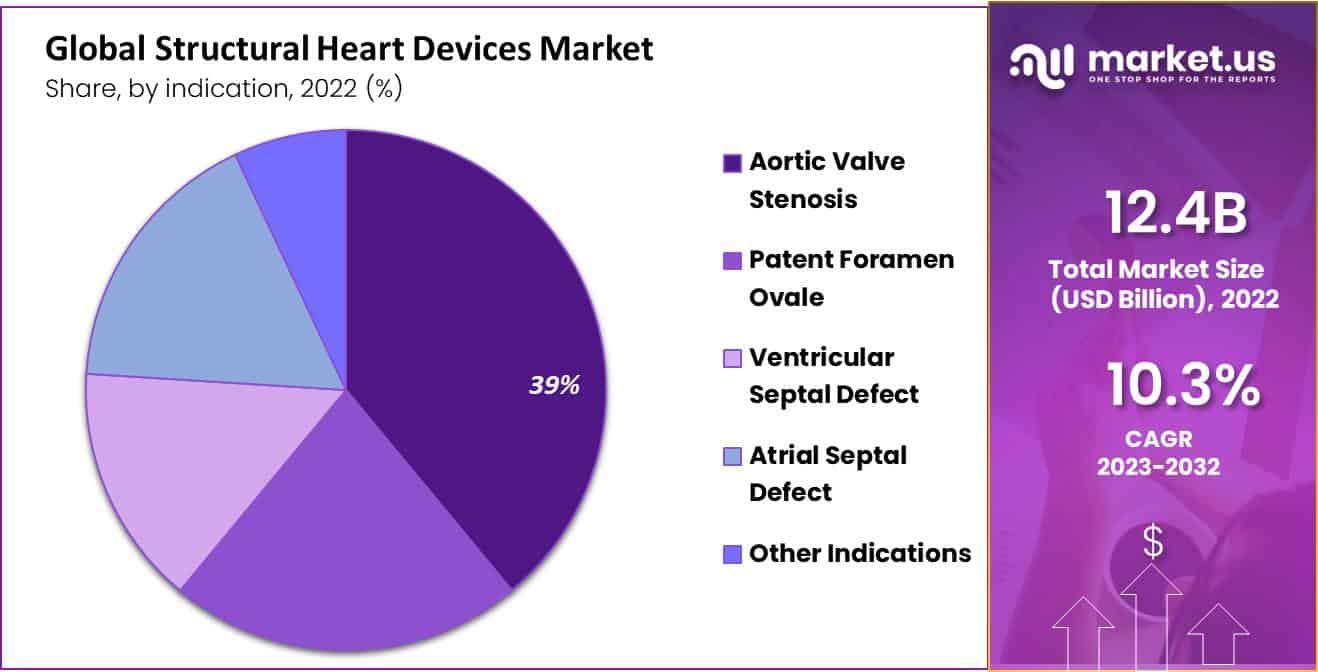
- The aortic valve stenosis segment accounted for the highest revenue share of 25% in 2022.
- Based on the procedure, the replacement procedures segment is predicted to hold the largest market share of 39% during the forecast period.
- Among end-users, the hospitals segment will see significant growth during the forecast period.
- The incidence rate of congenital heart defects is 1 in every 100 newborns.
- A SAPIEN transcatheter heart valve costs above $32,000.
- In the UK, over 7 million people are living with circulatory and heart diseases.
- Out of those 7 million people, over 3 million are females and nearly 4 million are males diagnosed with circulatory and heart diseases.
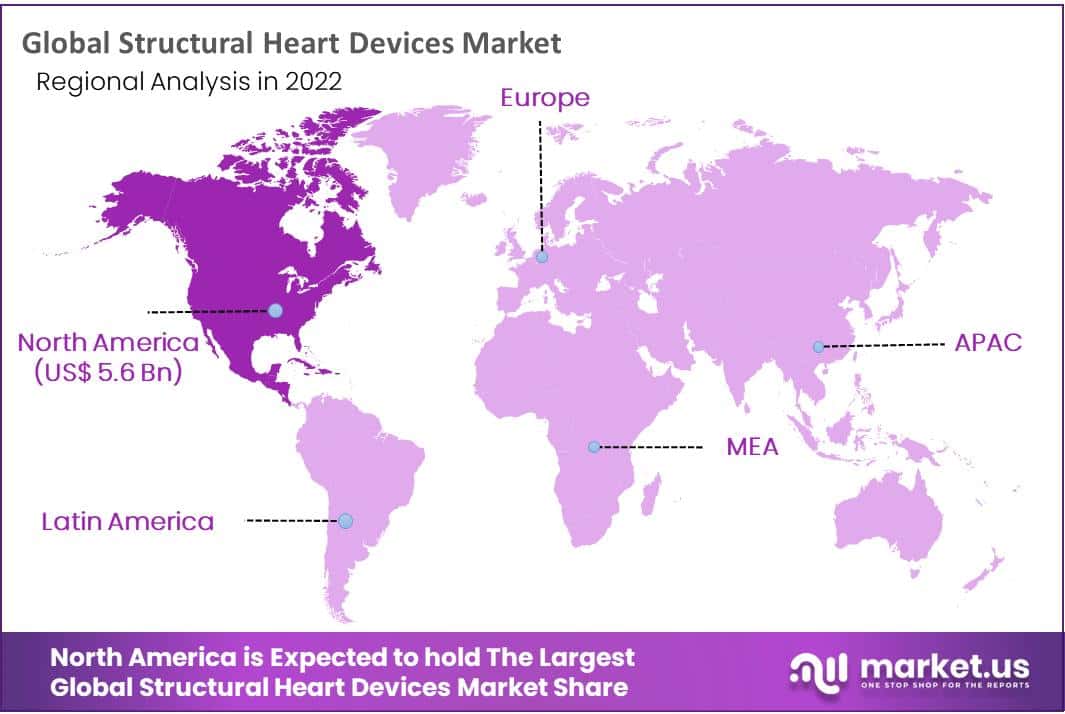
- During the forecast period, North America will dominate the global structural heart devices market with a revenue share of 45.3%.
- Abbott introduced a unique vascular heart valve repair device in May 2022.
Product Analysis
The Heart Valves Devices Segment Accounted for the Highest Share of the Market.
The structural heart devices are segmented into heart valve devices, annuloplasty rings, surgical heart valves, occluders and delivery systems, transcatheter heart valves, and other products based on products. During the forecast period, the heart valve devices segment is expected to hold the highest share of 34% of the market owing to the increasing number of transcatheter aortic valve replacement procedures performed around the globe.
In addition, the durability and efficacy of these products and the increasing number of regulatory approvals for heart valve diseases increase the demand for the segment.
Indication Analysis
Aortic Valve Stenosis Disease Will Dominate the Segment
The indication segment is divided into the atrial septal defect, patent foramen ovale, ventricular septal defect, aortic valve stenosis, and other indications. The aortic valve stenosis segment accounted for the highest revenue share of 25% in 2022. Aortic valve stenosis disease is associated with the aorta. The aorta is the artery that carries oxygenated blood from the heart to the body.
As a result, the aorta cannot open fully in the aortic valve stenosis. This disease is prevalent among the population. It is treated with the help of minimally invasive procedures by treating structural heart devices. Thus, aortic valve stenosis disease will dominate the segment.
Procedure Analysis
The Replacement Procedures dominated the Market
In the Structural Heart Devices Market, the procedure segmentation includes several key categories: replacement procedures, repair procedures, TAVR (Transcatheter Aortic Valve Replacement) procedures, SAVR (Surgical Aortic Valve Replacement) procedures, valvuloplasty, annuloplasty, and TMVR (Transcatheter Mitral Valve Repair) procedures. Among these, the replacement procedures segment is projected to hold the largest market share, estimated at 39% in 2023.
This dominance is indicative of a significant demand for these procedures, which are critical for treating various structural heart conditions effectively. The prominence of the replacement procedures segment highlights its essential role in addressing the needs of patients requiring heart valve replacements, reflecting advancements in medical technologies and surgical techniques that enhance patient outcomes.
End-User Analysis
Among End-Users Hospitals Segment Is Dominant
The end-user segment is divided into hospitals, ambulatory surgical centers, and other end-users. The hospital segment is estimated to record significant growth during the forecast period owing to the adoption of advanced technology in the hospitals and ongoing enhancements in the healthcare infrastructure.
Key Market Segments
Based on Product
- Heart Valve Devices
- Annuloplasty Rings
- Surgical Heart Valves
- Occluders and Delivery Systems
- Transcatheter Heart Valves
- Other Products
Based on Indication
- Atrial Septal Defect
- Patent Foramen Ovale
- Ventricular Septal Defect
- Aortic Valve Stenosis
- Other Indications
Based on Procedure
- Replacement Procedures
- Repair Procedures
- TAVR Procedures
- SAVR Procedures
- Valvuloplasty
- Annuloplasty
- TMVR Procedures
Based on End-User
- Hospitals
- Ambulatory Surgical Centers
- Other End-Users
Drivers
The Increasing Incidence of Structural Heart Diseases
There is an increase in demand for structural heart devices due to the increase in patients suffering from structural heart diseases. These diseases are congenital and generally very common in newborns. Micro Interventional Devices, Inc. stated that structural heart defects affected around 60 million people in the US.
As per the report of The Nemours Foundation, around 1 in every 100 newborns suffers from congenital heart defects, which may range from mild to severe. These statistics indicate the need for structural heart treatment devices to provide stimulus to develop new techniques for heart replacement and defect repair.
Trend
Minimally Invasive Procedures
A significant trend in the Structural Heart Devices Market is the shift towards minimally invasive procedures. Technologies such as Transcatheter Aortic Valve Replacement (TAVR) are becoming more popular due to their advantages over traditional surgical methods, including reduced recovery times and lower risk of complications.
This trend is supported by ongoing technological advancements that improve the safety and efficacy of minimally invasive techniques, making them more appealing to both healthcare providers and patients.
Restraints
The Procedures Are Dangerous and Expensive
The risks associated with procedures and the costly advanced structural heart devices significantly affect the market’s growth. Additionally, various risks associated with these devices include kidney failure, gastrointestinal complications, and stroke, observed following mitral valve replacement surgery.
In emerging countries, the adoption of technologically advanced structural heart devices is low. The reason behind this is the cost of these devices. Edwards Lifesciences stated that a SAPIEN transcatheter heart valve, including the RetroFlex 3 Transfemoral System, costs above $32,000. Such an amount is very costly for the average person.
Opportunities
High Prevalence of Heart and Circulatory Disease Patients
The major factors responsible for the market’s growth are the increasing significance of cardiovascular diseases globally, increasing availability of reimbursement policies, and increasing R&D & investments in efficient drug development.
For example, ‘The British Heart Foundation’ published ‘UK Factsheet 2022’; according to this report, in the UK, over 7 million people are living with circulatory and heart diseases, and over 3 million females and nearly 4 million males are diagnosed with circulatory and heart disease patients in the country.
The significance of circulatory and heart disease patients generates a high demand for treatment and procedures such as heart surgery and angioplasty for the survival of patients. Thus, it creates high opportunities for structural devices such as annuloplasty rings and heart valves worldwide.
Regional Analysis
North America Dominates the Global Structural Heart Devices Market During the Forecast Period
North America held the dominant revenue share of 45.3% for the global structural devices market. The major drivers of the market are the existence of a large population that suffers from structural heart diseases, technological advancements, the development of innovative devices, and others. In addition, with the help of well-established healthcare infrastructure, the early detection of structural heart diseases is made possible.
On the other hand, Asia Pacific is estimated to have the fastest growth during the forecast period. This is due to the rising geriatric population, increasing regulatory approvals, implementation of government-funded insurance scheme, increasing medical tourism industry in various APAC countries, favorable reimbursement scenario, better new-born screening programs, development in healthcare access, and rising awareness about cardiac diseases.
Key Regions and Countries
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Johnson & Johnson Announced its Intentions to Enter the Market
As new players enter the market, the industry has stable growth. For instance, in May 2016, Johnson & Johnson, a leading medical devices company, announced its goal to enter the structural heart market. Despite that, various new entrances and current players are strengthening their positions in the market by coming into various acquisitions & mergers.
Market Key Players
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Abbott
- LivaNova PLC
- Medtronic
- JenaValve Technology, Inc.
- Comed BV
- CardioKinetix
- Other Key Players
Key Industry developments
- Boston Scientific Corporation (June 2024): Acquired Silk Road Medical, Inc., which specializes in stroke prevention technologies, adding minimally invasive products to their vascular portfolio
- Edwards Lifesciences Corporation (2024): Acquired JenaValve Technology, enhancing its portfolio in transcatheter treatment for aortic regurgitation. Additionally, it acquired Endotronix, a leader in heart failure management, expanding Edwards’ structural heart portfolio
- JenaValve Technology, Inc. (2024): Acquired by Edwards Lifesciences, JenaValve contributes its expertise in aortic valve disease treatment, specifically focusing on addressing unmet needs in aortic regurgitation.
Report Scope
Report Features Description Market Value (2023) USD 13.7 Billion Forecast Revenue (2032) USD 32.2 Billion CAGR (2023-2032) 10.3% Base Year for Estimation 2023 Historic Period 2016-2022 Forecast Period 2024-2032 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Product- Heart Valve Devices, Annuloplasty Rings, Surgical Heart Valves, Occluders and Delivery Systems, Transcatheter Heart Valves, and Other Products; By Indication- Atrial Septal Defect, Patent Foramen Ovale, Ventricular Septal Defect, Aortic Valve Stenosis, and Other Indications; By Procedure- Replacement Procedures, Repair Procedures, TAVR Procedures, SAVR Procedures, Valvuloplasty, Annuloplasy, and TMVR Procedures; and By End-User- Hospitals, Ambulatory Surgical Centers, and Other End-Users. Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; The Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA. Competitive Landscape Boston Scientific Corporation, Edwards Lifesciences Corporation, Abbott, LivaNova PLC, Medtronic, JenaValve Technology, Inc., Comed BV, CardioKinetix, and Other Key Players. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Structural Heart Devices MarketPublished date: Nov 2023add_shopping_cartBuy Now get_appDownload Sample
Structural Heart Devices MarketPublished date: Nov 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Abbott
- LivaNova PLC
- Medtronic
- JenaValve Technology, Inc.
- Comed BV
- CardioKinetix
- Other Key Players










