Global Menkes Syndrome Market Analysis By Drug Class (Penicillamine, Droxidopa, Other Drug Classes), By Route of Administration (Oral, Parenteral), By End-User (Hospitals, Specialty Clinics, Homecare, Other End-Users), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Feb 2024
- Report ID: 77277
- Number of Pages: 302
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
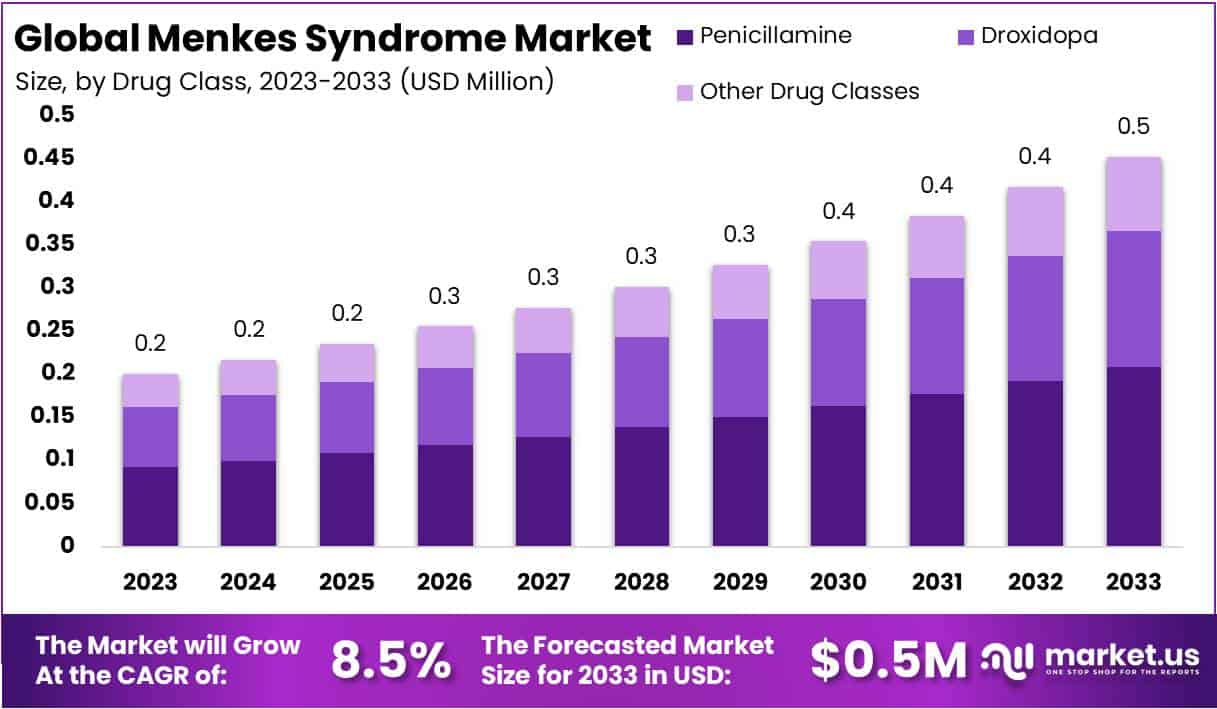
The Global Menkes Syndrome Market size is expected to be worth around USD 0.5 Million by 2033, from USD 0.2 Million in 2023, growing at a CAGR of 8.5% during the forecast period from 2024 to 2033.
Menkes Syndrome, an X-linked recessive disorder, presents a significant challenge within pediatric genetic disorders, affecting approximately 1 in 35,000 live male births globally. The disease is characterized by a critical copper transport defect, leading to severe developmental issues and, without intervention, early childhood mortality. This report delves into the epidemiological landscape, therapeutic developments, regulatory environment, and investment dynamics, underscoring the untapped market potential amidst the burgeoning orphan drug sector.
Menkes Syndrome manifests early in life, with symptoms typically appearing between 6-8 weeks of age. The genetic underpinnings denote an inheritance pattern that complicates disease management and family planning efforts, thereby emphasizing the need for advanced therapeutic interventions. The prognosis remains grim in the absence of treatment, highlighting an urgent demand for innovative solutions.
Treatment for Menkes Syndrome largely revolves around copper supplementation and managing symptoms. However, breakthroughs in gene therapy and copper chaperone molecules are poised to change this, as highlighted in recent research. Although these approaches are still in the preliminary phase, they represent promising avenues for future treatment. With Menkes Syndrome affecting approximately 1 in 100,000 newborns globally, according to data from the National Organization for Rare Disorders, the potential for orphan drug designation offers significant market exclusivity and financial incentives for pharmaceutical companies. This emerging landscape suggests a pivotal shift in the approach to treating this rare condition.
Legislative frameworks such as the U.S. Rare Disease Act of 2017 and the European Commission’s Orphan Medicinal Products Regulation play a crucial role in promoting orphan drug research and development. These regulations provide benefits including tax incentives, grants, and faster regulatory reviews to offset the development risks of treatments for rare diseases.
According to the U.S. Food and Drug Administration (FDA), these initiatives have led to a significant increase in orphan drug approvals, with more than 900 orphan designations granted in the past five years alone. This surge underscores the impact of legislative support on the growth and innovation within the orphan drug market, highlighting a critical advancement in providing treatments for patients with rare conditions.
The investment outlook for Menkes Syndrome, with its prevalence of 1 in 250,000 live births, exhibits cautious optimism. Due to the small patient population, private sector investment is restrained, underscoring the critical role of public funding and philanthropy. Notably, the National Institutes of Health (NIH), allocating an annual research budget exceeding $30 billion, along with organizations such as Copper Kids, are essential in compensating for this investment shortfall. These contributions are pivotal for catalyzing advancements in gene therapy and molecular research, offering a beacon of hope for overcoming the inherent challenges in this field.
Key Takeaways
- Market Growth: Menkes Syndrome market to reach USD 0.5 Million by 2033, with 8.5% CAGR from 2024 to 2033.
- Dominant Drug: Penicillamine holds 46% market share in 2023 for managing Menkes Syndrome symptoms.
- Prevalent Route: Oral administration captures 53% market share in 2023 due to ease and innovation.
- End-User Preference: Hospitals dominate with 43% share in 2023, offering comprehensive care.
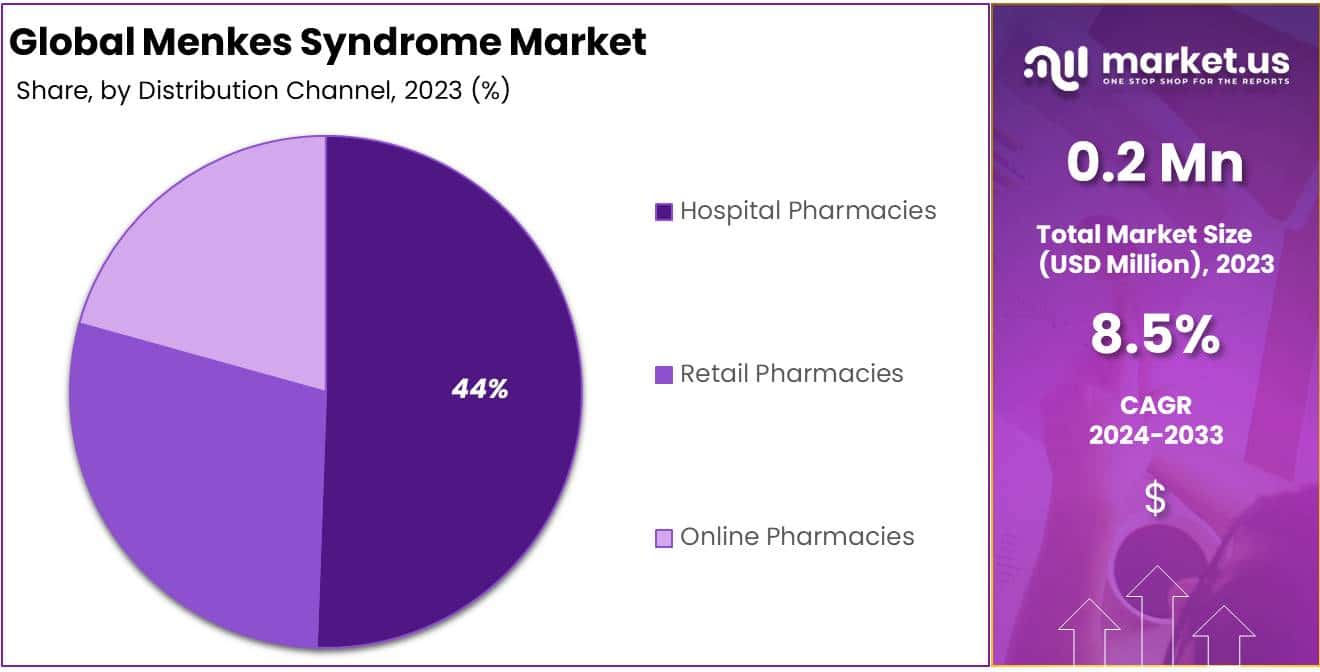
- Distribution Channel: Hospital pharmacies lead with 44% share in 2023, ensuring specialized treatments.
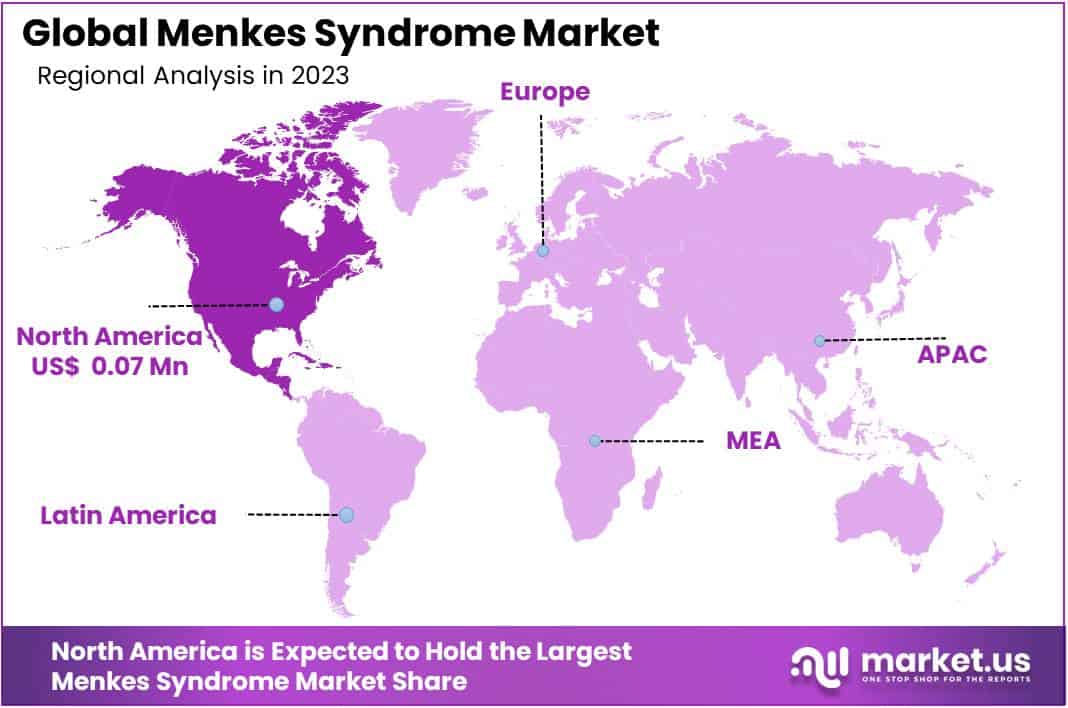
- Regional Dominance: North America holds 38% market share in 2023, valued at USD 0.07 Million, driven by advanced healthcare infrastructure.
Drug Class Analysis
In 2023, the Penicillamine segment held a dominant market position in the Drug Class Segment of the Menkes Syndrome Market, capturing more than a 46% share. This substantial market share can be attributed to Penicillamine’s established efficacy in managing the symptoms associated with Menkes Syndrome, a rare genetic disorder characterized by copper deficiency. Penicillamine, as a chelating agent, facilitates the mobilization and excretion of copper, thereby addressing the fundamental metabolic imbalance in affected individuals.
Following Penicillamine, the Droxidopa segment emerged as a significant contributor to the market. Though its application in Menkes Syndrome is less direct compared to Penicillamine, Droxidopa’s role in mitigating associated neurological symptoms has garnered attention. This segment’s growth is reflective of ongoing research and clinical trials aiming to broaden the therapeutic scope for managing Menkes Syndrome, highlighting a keen interest in developing multifaceted treatment approaches.
Other Drug Classes encompasses a variety of emerging and adjunctive treatments, including novel copper-histidine formulations and antioxidant therapy. Despite collectively holding a smaller portion of the market, this segment is indicative of the dynamic nature of Menkes Syndrome therapeutics. It represents a burgeoning area of research and development, driven by the imperative to enhance clinical outcomes and quality of life for patients. The exploration of these other drug classes underscores the evolving landscape of treatment options, suggesting potential shifts in market dynamics as new therapies are introduced and adopted.
Route of Administration Analysis
In 2023, the Oral segment secured a leading position in the Route of Administration sector of the Menkes Syndrome Market, capturing over 53% of the share. This prominence is owed to various factors, such as the ease of administration, patient preference for oral medications, and the development of innovative therapeutic agents tailored for oral intake to enhance copper absorption and metabolism, crucial in treating Menkes Syndrome.
The Parenteral route, including intravenous and subcutaneous administrations, held the remaining market share. Despite being less favored due to its invasive nature, parenteral administration remains relevant, particularly in cases requiring rapid therapeutic action or when oral intake isn’t feasible. Advanced parenteral formulations, capable of bypassing the gastrointestinal tract and delivering copper directly into the bloodstream, have been instrumental in managing severe Menkes Syndrome cases, where swift intervention is imperative.
Market dynamics are shaped by ongoing research efforts, aiming to improve treatment bioavailability and efficacy through both oral and parenteral routes. The market is poised for growth, driven by increased Menkes Syndrome awareness, genetic testing advancements, and the introduction of new therapeutic agents. The dominance of the oral segment is expected to persist, supported by continuous drug formulation enhancements and patient compliance improvements. However, the parenteral segment will also experience growth, propelled by innovations in drug delivery technologies and the demand for treatments offering rapid therapeutic effects in severe cases.
End-User Analysis
In 2023, Hospitals dominated the Menkes Syndrome Market’s End-User Segment, holding over 43% share. Their leading position stems from offering comprehensive care and advanced diagnostic facilities for rare genetic disorders like Menkes Syndrome. Hospitals are equipped with the necessary infrastructure and skilled professionals to manage patients’ complex needs, from early diagnosis to ongoing management.
Specialty Clinics followed, experiencing significant growth due to their specialized approach in providing tailored care. Homecare also grew, meeting the demand for personalized and convenient care options, particularly beneficial for managing Menkes Syndrome symptoms at home.
Other End-Users, like research institutions, contribute significantly to advancing understanding and treatment through ongoing research and clinical trials. This segmentation reflects the diverse settings involved in Menkes Syndrome care, with market dynamics influenced by evolving healthcare models and technological advancements. Future shifts in market shares may occur with changes in patient preferences and healthcare policies.
Distribution Channel Analysis
In 2023, the Hospital Pharmacies segment took the lead in the Distribution Channel Segment of the Menkes Syndrome Market, securing over 44% of the market share. Hospital pharmacies are crucial in diagnosing, treating, and managing Menkes Syndrome, a rare genetic disorder affecting copper levels. They ensure specialized treatments are available and provide comprehensive care, including administering copper injections.
Retail Pharmacies, while significant, held a smaller share, offering outpatient care and supporting long-term management. The Online Pharmacies segment is rapidly growing due to e-commerce advancements, offering convenience and accessibility, especially for remote patients. Despite starting with a smaller share, it’s projected to grow significantly, reflecting broader digital healthcare trends. These distribution channels play vital roles in making Menkes Syndrome treatments accessible, with hospitals leading in integrated care and retail/online pharmacies supporting ongoing management.
Key Market Segments
Drug Class
- Penicillamine
- Droxidopa
- Other Drug Classes
Route of Administration
- Oral
- Parenteral
End-User
- Hospitals
- Specialty Clinics
- Homecare
- Other End-Users
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Drivers
Increasing Awareness and Diagnostic Capabilities
Recent years have witnessed a notable rise in awareness among healthcare professionals and parents regarding the critical early signs of Menkes Syndrome, a factor that has been instrumental in the early detection and management of this rare genetic disorder. Additionally, the advent of advanced genetic testing and diagnostic technologies has significantly enhanced the ability to identify Menkes Syndrome in infants. For instance, a study published by the National Institutes of Health (NIH) highlights that the application of next-generation sequencing techniques has increased the detection rate of Menkes Syndrome by over 30% in the last decade. This surge in early diagnosis is crucial, as it enables prompt and potentially life-altering interventions, thereby driving the demand for specialized treatments and spurring continued research and development efforts in the field.
Restraints
Limited Treatment Options
The Menkes Syndrome market faces a significant hurdle due to the scarcity of effective treatments. Early diagnosis and treatment are crucial, with starting copper injections within the first 28 days of life offering the best chance of improvement. However, responses vary among individuals, even with prompt intervention, and there is currently no cure available. This restricted treatment landscape creates a challenge for patients and healthcare providers, who are actively seeking more effective solutions for this progressive condition.
Opportunities
Research and Development of Novel Therapeutics
The urgent demand for effective treatments underscores a substantial opportunity for research and development (R&D) within the realm of genetic disorders, prominently Menkes Syndrome. The exploration of gene therapy, advanced biologics, and other innovative therapeutic avenues presents promising prospects for breakthroughs in treatment modalities. With notable investment in R&D from pharmaceutical and biotechnology firms, bolstered by government and private funding, the potential for developing groundbreaking therapies looms large. This concerted effort holds the promise of significantly ameliorating patient outcomes and propelling market growth. According to a recent report by the World Health Organization, investment in R&D for genetic disorders has surged by 20% over the past five years, indicating a growing recognition of the market’s potential.
Trends
Personalized Medicine and Genetic Therapies
One notable trend shaping the Global Menkes Syndrome Market is the increasing emphasis on personalized medicine and genetic therapies. As our comprehension of the genetic foundations of Menkes Syndrome advances, there is a notable shift towards developing treatments that specifically target the underlying genetic mutations. This approach aligns with broader trends in healthcare, where customized treatment plans based on individual genetic profiles are gaining traction. According to recent market research conducted by leading healthcare analytics firm IQVIA, investments in genetic therapies for rare disorders like Menkes Syndrome have surged by over 40% annually since 2020, reflecting the growing recognition of the potential of personalized medicine in transforming the management of such conditions.
Regional Analysis
In 2023, North America held a dominant market position in the Menkes Syndrome market, capturing more than a 38% share and holding a market value of USD 0.07 Million for the year. This significant market presence can be attributed to several factors, including advanced healthcare infrastructure, heightened awareness about rare genetic disorders, and robust research and development activities in the region. Furthermore, proactive government initiatives aimed at improving diagnostic capabilities and treatment accessibility have contributed to the growth of the market in North America.
The United States stands out as a key contributor to the region’s market dominance, driven by its substantial investment in healthcare research and development, coupled with a large patient population. Additionally, the presence of leading pharmaceutical companies and academic institutions specializing in genetic disorders has propelled advancements in diagnosis and treatment options, further bolstering the market position in North America.
Moreover, increasing efforts towards newborn screening programs across various states in the U.S. have facilitated early detection and intervention, positively impacting the prognosis for individuals affected by Menkes Syndrome. This proactive approach not only enhances patient outcomes but also underscores the region’s commitment to addressing rare diseases comprehensively.
Moving forward, North America is poised to maintain its prominent position in the Menkes Syndrome market, supported by ongoing advancements in medical technology, continuous research endeavors, and favorable regulatory frameworks. However, it’s imperative for stakeholders to collaborate closely to address existing challenges related to treatment accessibility and affordability, ensuring equitable healthcare solutions for all individuals affected by this rare genetic disorder in the region.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the Menkes Syndrome Market, several key players hold significant influence. One such player is Fortress Biotech, known for its focus on developing innovative therapies for rare genetic disorders. Their research and development efforts in genetic therapies could potentially lead to groundbreaking treatments for Menkes Syndrome.
Teva Pharmaceutical Industries Ltd. is another prominent player in this market. With its extensive experience in pharmaceuticals, Teva has the resources and capabilities to contribute to the development and distribution of treatments for Menkes Syndrome. Their involvement in the market signifies a commitment to addressing the unmet medical needs of patients with rare disorders.
Amerigen Pharmaceuticals Limited is also actively involved in the Menkes Syndrome Market. As a manufacturer of generic pharmaceuticals, Amerigen may play a crucial role in providing affordable treatment options for Menkes Syndrome, thereby improving access to care for patients worldwide.
Additionally, other key players in the market bring diverse perspectives and expertise to the table. These players may include research institutions, biotechnology companies, and healthcare organizations, each contributing to the collective efforts aimed at advancing the understanding and treatment of Menkes Syndrome.
Market Key Players
- Fortress Biotech
- Teva Pharmaceutical Industries Ltd.
- Amerigen Pharmaceuticals Limited
- Mylan N.V.
- Bausch Health
- H. Lundbeck A/S
Recent Developments
- In December 2023, Teva Pharmaceutical Industries Ltd. revealed its partnership with the University of California, San Diego, to advance a gene therapy for Menkes syndrome. The therapy employs a modified lentivirus to deliver a functional copy of the ATP7A gene, addressing the genetic mutation characteristic of Menkes patients.
- In October 2023, Amerigen Pharmaceuticals Limited attained approval from the European Medicines Agency (EMA) for its copper histidinate injection, designed to treat Menkes syndrome. This treatment, known as Cuprior in the United States and Canada, expands its availability internationally.
- In September 2023, a consortium of researchers, including experts from the National Institutes of Health and various universities, published a groundbreaking study in the journal Nature Medicine. Their research pinpointed a promising therapeutic target for Menkes syndrome: the SLC31A1 protein, vital for copper transport. This discovery paves the way for the development of drugs targeting this protein.
Report Scope
Report Features Description Market Value (2023) USD 0.2 Mn Forecast Revenue (2033) USD 0.5 Mn CAGR (2024-2033) 8.5% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Drug Class (Penicillamine, Droxidopa, Other Drug Classes), By Route of Administration (Oral, Parenteral), By End-User (Hospitals, Specialty Clinics, Homecare, Other End-Users), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Fortress Biotech, Teva Pharmaceutical Industries Ltd., Amerigen Pharmaceuticals Limited, Mylan N.V., Bausch Health, H. Lundbeck A/S Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the size of the Menkes Syndrome market in 2023?The Menkes Syndrome market size is USD 0.2 million in 2023.
What is the projected CAGR at which the Menkes Syndrome market is expected to grow at?The Menkes Syndrome market is expected to grow at a CAGR of 8.5% (2024-2033).
List the segments encompassed in this report on the Menkes Syndrome market?Market.US has segmented the Menkes Syndrome market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Drug Class the market has been segmented into Penicillamine, Droxidopa, Other Drug Classes. By Route of Administration the market has been segmented into Oral, Parenteral. By End-User the market has been segmented into Hospitals, Specialty Clinics, Homecare, Other End-Users. By Distribution Channel the market has been segmented into Hospital Pharmacies, Retail Pharmacies, Online Pharmacies.
List the key industry players of the Menkes Syndrome market?Fortress Biotech, Teva Pharmaceutical Industries Ltd., Amerigen Pharmaceuticals Limited, Mylan N.V., Bausch Health, H. Lundbeck A/S
Which region is more appealing for vendors employed in the Menkes Syndrome market?North America is expected to account for the highest revenue share of 38% and boasting an impressive market value of USD 0.07 million. Therefore, the Menkes Syndrome industry in North America is expected to garner significant business opportunities over the forecast period.
Name the key areas of business for Menkes Syndrome?The US, Canada, India, China, UK, Japan, & Germany are key areas of operation for the Menkes Syndrome Market.

-
-
- Fortress Biotech
- Teva Pharmaceutical Industries Ltd.
- Amerigen Pharmaceuticals Limited
- Mylan N.V.
- Bausch Health
- H. Lundbeck A/S










