Global Macular Edema and Macular Degeneration Market By Treatment Type (Drug Therapy, Laser Treatment) By Application (Macular Edema, Diabetic Macular Edema (DME), Cystoid Macular Edema (CME), Macular Degeneration, Dry age-related macular degeneration, Wet age-related macular degeneration) By End User, Hospitals, Clinics, Others) Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: June 2025
- Report ID: 150176
- Number of Pages: 244
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
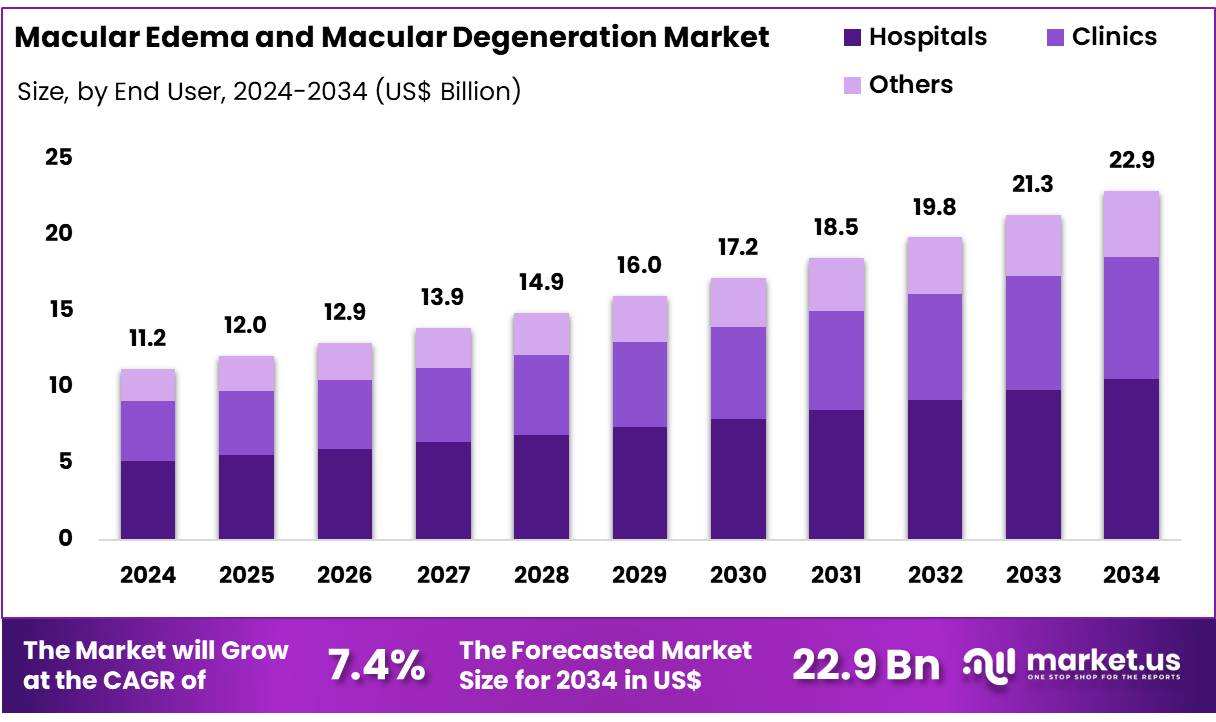
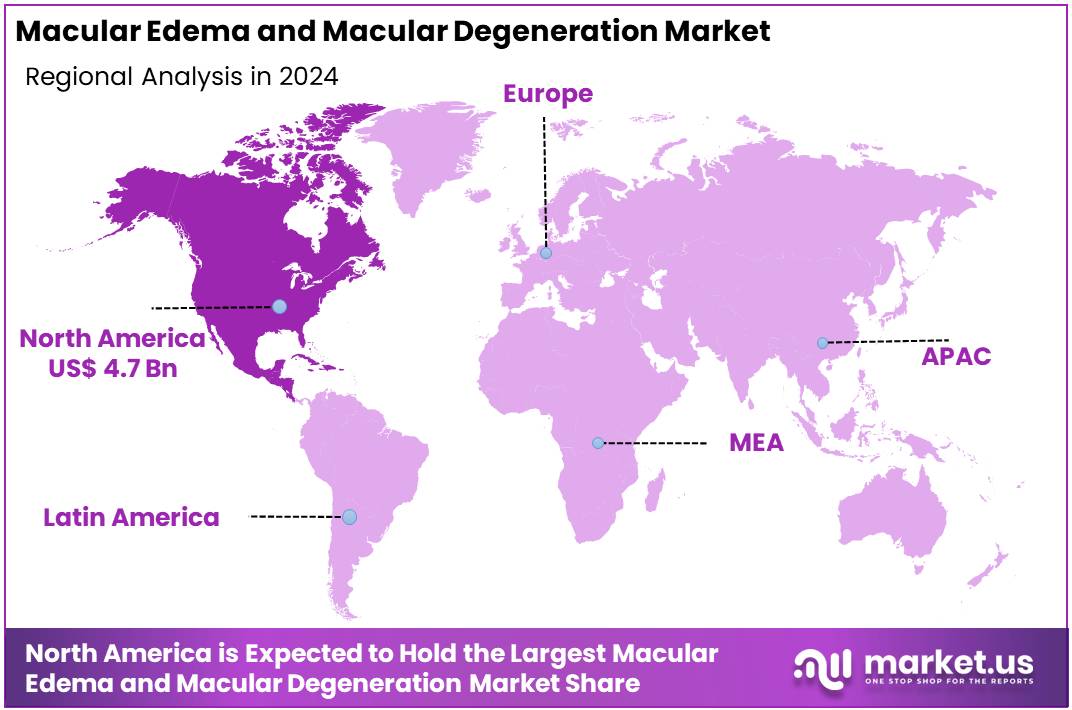
Global Macular Edema and Macular Degeneration Market size is expected to be worth around US$ 22.9 Billion by 2034 from US$ 11.2 Billion in 2024, growing at a CAGR of 7.2% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 42.7% share with a revenue of US$ 4.7 Billion.
Macular edema and macular degeneration are serious retinal conditions that can lead to vision impairment and blindness if untreated. Macular edema occurs when leaky blood vessels cause fluid to accumulate in the macula, the central area of the retina responsible for sharp vision. Diabetic retinopathy is the most common cause of macular edema; approximately 4–7% of people with diabetes have diabetic macular edema.
Other causes include retinal vein occlusion, uveitis, and postoperative inflammation. Diagnosis relies on a comprehensive eye exam, optical coherence tomography, and fluorescein angiography to detect retinal swelling.In contrast, age-related macular degeneration (AMD) involves progressive deterioration of the macula due to aging and genetic factors.
Globally, AMD affects roughly 8% of the population and around 11 million Americans. Dry AMD, accounting for 85–90% of cases, is marked by drusen deposits under the retina and gradual vision loss. Wet AMD, more severe, features abnormal blood vessel growth that rapidly damages the macula.
Regular eye exams enable early detection. Current interventions, such as anti-VEGF injections for wet AMD and managing systemic risk factors for both conditions, can slow progression and preserve vision. Awareness and timely intervention remain critical to improving patient outcomes worldwide.
Key Takeaways
- Market Size: Global Macular Edema and Macular Degeneration Market size is expected to be worth around US$ 22.9 Billion by 2034 from US$ 11.02 Billion in 2024.
- Market Growth: The market growing at a CAGR of 7.2% during the forecast period from 2025 to 2034.
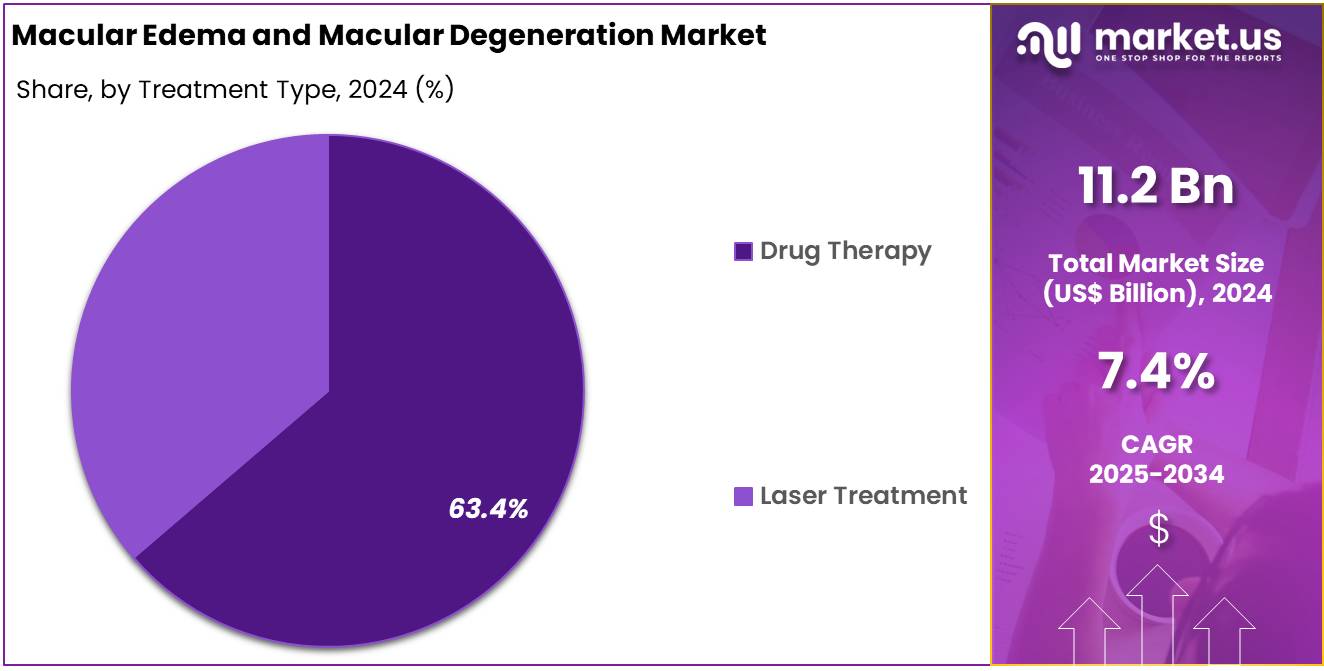
- Treatment Type Analysis: The drug therapy segment is responsible for a dominant market share of 63.4% in 2024.
- Application Analysis: The macular edema segment accounted for a dominant 29.3% share in 2024.
- End-Use Analysis: The hospital segment held a dominant 46.2% share in 2024.
- Regional Analysis: In 2024, North America led the market, achieving over 42.7% share with a revenue of US$ 4.7 Billion.
Treatment Type Analysis
The treatment type segmentation of the macular edema and macular degeneration market comprises two categories: drug therapy and laser treatment. The drug therapy segment is responsible for a dominant market share of 63.4% in 2024. This dominance can be attributed to the widespread adoption of anti-VEGF agents and corticosteroid implants, which have been validated by regulatory bodies for efficacy and safety.
The efficacy of pharmacologic interventions in reducing fluid accumulation and improving visual acuity has driven payer coverage and physician preference. Conversely, the laser treatment segment remains significant due to established use in focal and grid laser procedures for diabetic macular edema and photocoagulation in early-stage wet age-related macular degeneration.
However, laser procedures are being limited by advancements in pharmacotherapy, which offer minimally invasive administration routes and prolonged treatment intervals. As innovation continues, the drug therapy segment is expected to maintain leadership, while laser treatment is anticipated to be positioned as a complementary option for specific patient subgroups.
Application Analysis
In 2024, the application segmentation of the macular edema and macular degeneration market is divided into macular edema and macular degeneration subsegments. The macular edema segment accounted for a dominant 29.3% share in 2024. Within this segment, diabetic macular edema (DME) represents the largest portion due to rising diabetes prevalence and robust reimbursement for anti-VEGF treatments.
Cystoid macular edema (CME) follows, supported by advancements in corticosteroid injections. The macular degeneration segment is further divided into dry and wet age-related macular degeneration (AMD). Dry AMD holds a smaller share owing to limited therapeutic options but benefits from growing clinical trials targeting complement pathways and nutritional supplements.
Wet AMD maintains a substantial share driven by the high adoption of anti-VEGF agents and sustained-release implants. The influx of pipeline therapies in both dry and wet AMD is anticipated to notably reshape the segmental dynamics. Overall, macular edema remains the leading application in 2024, while AMD subsegments continue to exhibit significant growth potential consistently.
End User Analysis
The end-user segmentation of the macular edema and macular degeneration market comprises hospitals, clinics, and other healthcare settings. The hospital segment held a dominant 46.2% share, driven by robust ophthalmic care infrastructure and advanced diagnostic capabilities. Hospitals benefit from multidisciplinary teams, enabling seamless integration of intravitreal injections, laser procedures, and surgical interventions.
Reimbursement policies favoring inpatient and outpatient hospital services have contributed to elevated patient access. The clinic segment occupies a significant position, supported by the proliferation of specialized ophthalmology and retina clinics. These clinics offer focused patient management and increased frequency of follow-up visits, particularly for chronic conditions requiring regular anti-VEGF therapy.
Their emphasis on cost-effective ambulatory care solutions continues to attract patients preferring outpatient treatment. The remaining share is attributed to other end-users, including ambulatory surgery centers and academic research institutions. These entities facilitate clinical trials and novel therapy adoption, thereby influencing market dynamics. Overall, hospitals maintain leadership, while clinics and other settings demonstrate growth potential.
Key Market Segments
By Treatment Type
- Drug Therapy
- Laser Treatment
By Application
- Macular Edema
- Diabetic Macular Edema (DME)
- Cystoid Macular Edema (CME)
- Macular Degeneration
- Dry age-related macular degeneration
- Wet age-related macular degeneration
By End User
- Hospitals
- Clinics
- Others
Driving Factors
The macular edema and macular degeneration market is driven by the rising prevalence of age-related macular degeneration (AMD) and diabetic macular edema (DME), attributable to aging populations and increasing diabetes incidence. According to the National Eye Institute, AMD affects over 200 million people globally, with projections reaching 300 million by 2040, driven by demographic shifts toward older age groups.
Concurrently, diabetic retinopathy remains a leading cause of vision loss in diabetic patients, with DME occurring when retinal blood vessels leak into the macula, causing swelling and visual impairment, as noted by NEI. The resultant growth in patient numbers directly fuels market demand.
Trending Factors
The market is witnessing a transition toward advanced pharmacologic therapies, notably the widespread adoption of anti-VEGF agents (e.g., ranibizumab, aflibercept), which offer improved visual outcomes and reduced injection frequency. Recent NEI-funded research indicates potential new pharmacologic options, including repurposed antiretroviral drugs such as lamivudine for DME management.
Moreover, sustained-release intravitreal implants and gene therapy candidates are undergoing clinical evaluation, reflecting NIH investment in innovative interventions. Integration of artificial intelligence in diagnostic imaging has enhanced early detection and personalized treatment planning. This evolution is expected to continue as real-world evidence supports greater efficacy and tolerance.
Restraining Factors
High treatment costs and complex reimbursement policies limit market growth. Intravitreal injections of anti-VEGF agents require frequent administration, imposing significant financial burdens on patients and healthcare systems. For example, Medicare coding guidelines stipulate specific billing codes and modifiers for intravitreal injections, leading to procedural complexity and potential claim denials.
Additionally, limited access to specialized ophthalmic services in rural areas and emerging markets restricts patient reach. Safety concerns, such as risk of endophthalmitis and ocular hypertension, contribute to physician hesitancy and patient attrition. Laser treatment alternatives, while established, often yield suboptimal outcomes compared to pharmacologic options, reducing their appeal and utilization. Overall, market penetration is compromised.
Opportunity
The market presents significant opportunities driven by emerging therapies and expanding geographic reach. Pipeline candidates targeting dry AMD mechanisms—such as complement inhibitors and gene therapies may address unmet needs, as evidenced by NIH-supported clinical trials. Adoption of teleophthalmology platforms can facilitate remote screening and management, enhancing access in underserved regions.
Increasing healthcare expenditure in Asia-Pacific, combined with growing awareness of vision health, is anticipated to open new markets. Nutritional supplement formulations validated by AREDS2 data provide adjunctive treatment pathways, expanding product portfolios. Furthermore, reclassification of AMD as a priority health issue by the World Health Organization may stimulate funding and policy support.
Regional Analysis
In 2024, North America held a dominant market position, capturing more than a 72.7% share and holds US$ 4.7 Billion market value for the year. The region is characterized by a high prevalence of age-related macular degeneration (AMD). According to the National Eye Institute, AMD affects approximately one in ten Americans aged 50 and older.
Concurrently, diabetic macular edema (DME) impacts about 3.8% of U.S. adults aged ≥ 40 years with diabetes, equating to roughly 746,000 individuals. Robust healthcare infrastructure supports early diagnosis through widespread availability of OCT imaging and specialized retina clinics. Reimbursement policies under Medicare and private insurers favor intravitreal therapies, enabling higher treatment uptake.
Substantial research funding from NIH and NEI has fostered pipeline innovations in pharmacotherapy and gene therapy. Teleophthalmology platforms have expanded care into rural areas. Public health initiatives, such as Healthy People 2030, emphasize vision screening and awareness, further driving patient engagement. Together, these factors reinforce North America’s leadership, while ongoing efforts to improve access and fund novel treatments suggest continued dominance.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Market leaders are characterized by extensive anti-VEGF portfolios and strong R&D pipelines. These entities leverage robust clinical trial data to validate novel therapies. They possess advanced manufacturing facilities ensuring high-quality biologics. Geographic diversification allows rapid market entry across regions with varied reimbursement systems.
Strategic alliances with academic institutions and research consortia drive innovation in gene therapy and sustained-release formulations. Comprehensive ophthalmic diagnostic solutions, such as OCT platforms, complement treatment offerings and enhance clinician adoption. These organizations maintain extensive medical affairs teams to support educational initiatives and payer negotiations. Their financial strength supports sustained investment in next-generation therapeutics, reinforcing market influence and long-term growth potential.
Market Key Players
- Kubota Pharmaceutical Holdings Co. Ltd
- Alimera Sciences Inc.
- Abbvie Inc.
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Regeneron Pharmaceuticals Inc.
- Regen X Bio Inc.
- GlaxoSmithKline Plc
- Bayer AG
- Bausch Health Companies Inc.
Recent Developments
- Kubota Pharmaceutical Holdings Co. Ltd: In January 2024, Kubota Vision initiated a Phase I PBOS clinical study in patients with diabetic macular edema (DME), marking its first human trial for the investigational PBOS implant. The study aims to evaluate safety, tolerability, and preliminary efficacy of the device for sustained intravitreal drug delivery in DME. This development represents Kubota’s entry into therapeutic strategies targeting macular edema.
- AbbVie Inc.: In January 2025, AbbVie and Regeneron Pharmaceuticals Inc. announced plans for a Phase III clinical program to evaluate RGX-314, a one-time gene therapy candidate for wet AMD and DME. The collaboration follows positive interim data from Phase II studies and is intended to support global regulatory submissions. The program will utilize in-office SCS Microinjector® delivery.
- F. Hoffmann-La Roche Ltd: In July 2024, Genentech (a Roche subsidiary) reintroduced Susvimo® (ranibizumab implant) for wet age-related macular degeneration in the U.S. after resolving prior component-related issues. The FDA approved a post-approval supplement updating implant and refill-needle components. Susvimo delivers ranibizumab continuously, reducing injection burden to two refill procedures per year.
Report Scope
Report Features Description Market Value (2024) US$ 11.2 Billion Forecast Revenue (2034) US$ 22.9 Billion CAGR (2025-2034) 7.2% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Treatment Type (Drug Therapy, Laser Treatment) By Application (Macular Edema, Diabetic Macular Edema (DME), Cystoid Macular Edema (CME), Macular Degeneration, Dry age-related macular degeneration, Wet age-related macular degeneration) By End User, Hospitals, Clinics, Others Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape Kubota Pharmaceutical Holdings Co. Ltd, Alimera Sciences Inc., Abbvie Inc., Novartis AG, F. Hoffmann-La Roche Ltd, Regeneron Pharmaceuticals Inc., Regen X Bio Inc., GlaxoSmithKline Plc, Bayer AG, Bausch Health Companies Inc. Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Macular Edema and Macular Degeneration MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample
Macular Edema and Macular Degeneration MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Kubota Pharmaceutical Holdings Co. Ltd
- Alimera Sciences Inc.
- Abbvie Inc.
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Regeneron Pharmaceuticals Inc.
- Regen X Bio Inc.
- GlaxoSmithKline Plc
- Bayer AG
- Bausch Health Companies Inc.










