Global Intracranial Pressure Monitoring Devices Market By Product (Invasive, and Non-invasive), By Application, By End-User (Hospitals and Clinic, and Trauma Centers), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 102957
- Number of Pages: 225
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
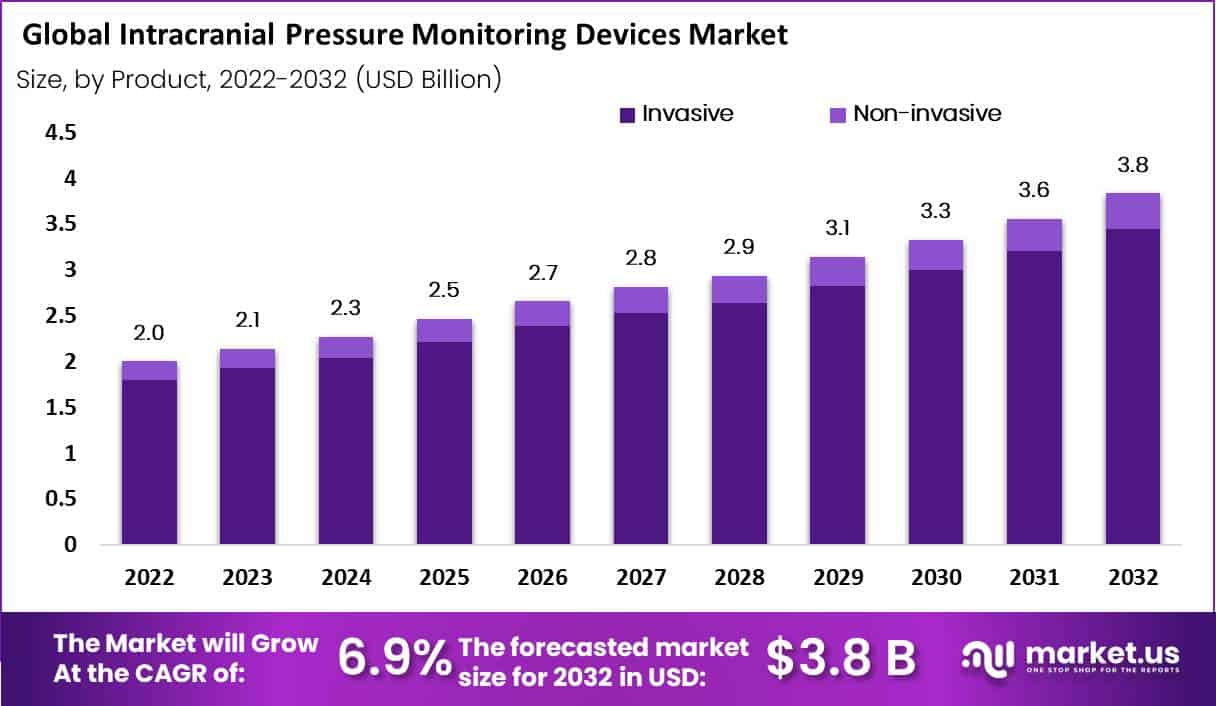
In 2022, the global intracranial pressure monitoring devices market accounted for USD 2.0 Billion and will reach USD 3.8 Billion by 2032. Between 2023 and 2032, this market is estimated to register a CAGR of 6.9%.
The intracranial pressure monitoring devices market refers to the industry that produces medical devices designed to measure & monitor the pressure inside the skull. These devices are typically used in critical care settings to help diagnose and manage conditions that affect the brain.
The market for these devices comprises invasive and non-invasive monitoring systems, as well as accessories and consumables utilized with these systems. The expanding number of persons affected by neurological illnesses, as well as the need for better medical technology to manage them, is driving up demand for these gadgets.
*Actual Numbers Might Vary In The Final Report
Key Takeaways
- Clinical Importance: Intracranial pressure monitoring is vital in diagnosing and managing patients suffering from brain injuries, intracranial hemorrhages, hydrocephalus or any neurological conditions requiring assessment or management – helping prevent potential brain damage by keeping an accurate account of ICP fluctuations in patients.
- Device Options: There are various ICP monitoring devices on the market, from external ventricular drains (EVDs), intraparenchymal monitors, subdural sensors and epidural sensors as well as noninvasive options available on the market to choose from. Your choice should depend upon individual clinical needs and patient conditions.
- Traumatic Brain Injuries: Traumatic brain injuries sustained through accidents are one of the primary drivers behind ICP monitoring device usage. Such devices play a vital role in monitoring and treating elevated ICP levels that could prove life-threatening.
- Neurosurgery and Neurology: ICP monitoring devices are frequently employed by both neurosurgical and neurological clinics to obtain real-time data to aid clinicians in making more informed decisions regarding treatment, surgery or interventions.
- Emergency Medicine: ICP monitoring may also be used as part of emergency care to evaluate patients with head injuries, helping direct medical teams in administering the appropriate care for each case.
- Neurocritical Care: Neurocritical care units rely heavily on ICP monitoring as part of their standard protocol for treating severe brain injuries or neurological diseases, to enable continuous assessment and intervention as needed.
Driving Factors
Increasing Incidence of Neurological Disorders and Technological Advancements
The development of the latest and most advanced intracranial pressure monitoring devices, including minimally invasive & wireless operating systems, is driving market growth. As the worldwide population continues to age, the incidence of neurological disorders like traumatic brain injury, stroke, as well hydrocephalus is increasing. This reason helps in leading to a higher demand for intracranial pressure monitoring devices.
The growing need for critical care facilities and services, especially in emerging nations, is propelling the intracranial pressure monitoring devices market forward. Various government efforts and regulations aiming at enhancing healthcare infrastructure and boosting access to healthcare services are propelling the industry forward. Globally, rising healthcare costs are pushing up demand for innovative medical technology such as intracranial pressure monitoring devices.
Restraining Factors
High Cost and Limited Reimbursement Policies
Intracranial pressure monitoring devices are often expensive, which may limit their adoption in certain regions or by certain patient populations. Limited or inadequate reimbursement policies for intracranial pressure monitoring procedures may also limit the demand for these devices.
Invasive intracranial pressure monitoring devices require the insertion of a catheter into the brain, which can be risky and may cause complications, leading to a limited adoption rate. The proper use and interpretation of intracranial pressure monitoring devices require specialized training, and the shortage of skilled professionals may hinder market growth.
By Product Analysis
The Invasive Segment Accounted for the Largest Revenue Share in Intracranial Pressure Monitoring Devices Market in 2022.
Based on product, the market is segmented into invasive, and non-invasive. The invasive category is expected to be the most profitable in the worldwide intracranial pressure monitoring devices market, accounting for 90% of total revenue during the forecast period.
To measure intracranial pressure, invasive technologies often entail inserting a catheter or sensor directly into the brain or cerebrospinal fluid. These devices are the gold standard for precise intracranial pressure monitoring, but they involve an intrusive procedure and pose the risk of infection or other problems.
By Application Analysis
The Traumatic Brain Injury Segment Holds the Significant Share of the Application Segment in the Intracranial Pressure Monitoring Devices Market.
Based on application, the market is divided into traumatic brain injury, intracerebral hemorrhage, meningitis, subarachnoid hemorrhage, CSF management, migraine, stroke, hydrocephalus, EEG, and other applications. Among these, the traumatic brain injury segment is dominant in the application segment in the intracranial pressure monitoring devices market, with a market share of 34%.
Traumatic brain injury is a common application for intracranial pressure monitoring devices, as elevated intracranial pressure is a common complication in patients with TBI. Monitoring intracranial pressure can help healthcare providers optimize treatment and prevent further brain damage.
By End-User Analysis
Hospitals and Clinics Hold a Significant Share in End-User Segment of Intracranial Pressure Monitoring Devices Market.
Based on end-user, the market is divided into hospitals and Clinics, and trauma centers. Among these, the hospitals and Clinics segment dominates the market with a revenue share of 78% in the forecasted period.
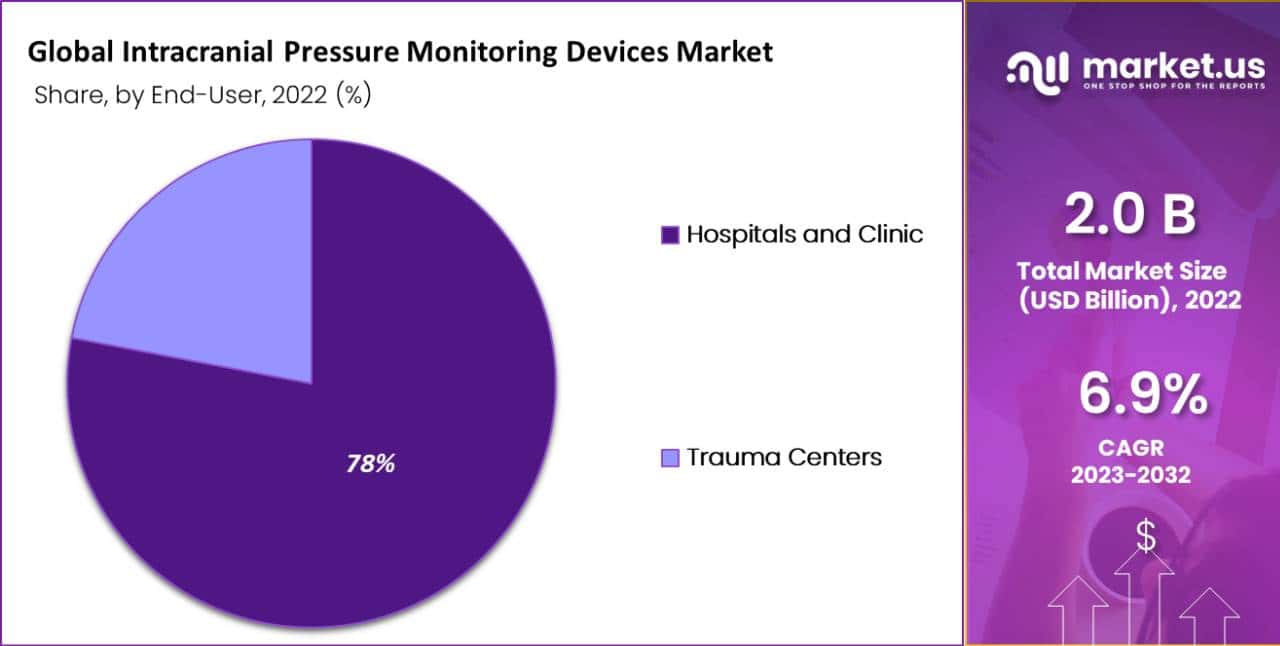
This is because hospitals and clinics are the most common end-users of intracranial pressure monitoring devices. These facilities provide a range of medical and surgical services and often have specialized neurocritical care units that manage patients with severe brain injuries or neurological conditions.
Market Segments
Based on Product
-
Invasive
-
- Microtransducer ICP Monitoring
- External Ventricular Drainage (EVD)
-
Non-invasive
-
- Transcranial Doppler Ultrasonography
- Tympanic Membrane Displacement (TMD)
- Optic Nerve Sheath Diameter
- MRI/CT
- Fundoscopy (papilledema)
Based on Application
- Traumatic Brain Injury
- Intracerebral Hemorrhage
- Meningitis
- Subarachnoid Hemorrhage
- CSF Management
- Migraine
- Stroke
- Hydrocephalus
- EEG
- Other Applications
Based on End-User
- Hospitals and Clinic
- Trauma Centers
Growth Opportunity
Emerging Markets and Home-Based Monitoring
Growth prospects in the intracranial pressure monitoring devices market provide industry participants with the opportunity to develop their businesses and boost their market share. These possibilities may develop as a result of several circumstances such as technical breakthroughs, increased demand for innovative medical technology, and growing markets.
For example, as technology advances, market participants can create more advanced, accurate, and user-friendly intracranial pressure monitoring systems capable of real-time functioning and data processing. This might assist healthcare workers in making numerous educated decisions about patient care, resulting in improved patient outcomes and greater demand for these devices.
Emerging markets provide market participants with the potential to grow their operations by tapping into regions with increasing demand for critical care services and improved healthcare infrastructure.
Latest Trends
Development and Increasing Use of Wireless Monitoring Devices.
Home-based monitoring devices offer patients greater flexibility and convenience while reducing the burden on healthcare facilities. Market players can capitalize on this trend by developing devices that are easy to use and offer reliable and accurate data.
Another rising trend is the creation of non-invasive monitoring devices, which offer an alternative to intrusive therapies that might jeopardize patients. This advancement is especially significant for patients who require long-term monitoring, as non-invasive solutions offer greater comfort and convenience. Another development in the intracranial pressure monitoring devices market is an increasing emphasis on patient safety.
Market participants are working on technologies that reduce the risk of infection and other consequences connected with invasive operations. This trend is predicted to continue as healthcare providers prioritize patient safety and infection control measures. Finally, as a result of the COVID-19 outbreak, there is a growing need for home-based monitoring systems.
Regional Analysis
North America Accounted for the Largest Revenue Share in Intracranial Pressure Monitoring Devices Market in 2022.
North America will be the dominant region in the global Intracranial Pressure Monitoring Devices Market. The region’s dominance is attributed to factors such as a high prevalence of traumatic brain injuries, the presence of leading market players, and favorable reimbursement policies. The US is the largest market in the region, with Canada also contributing significantly to the market’s growth.
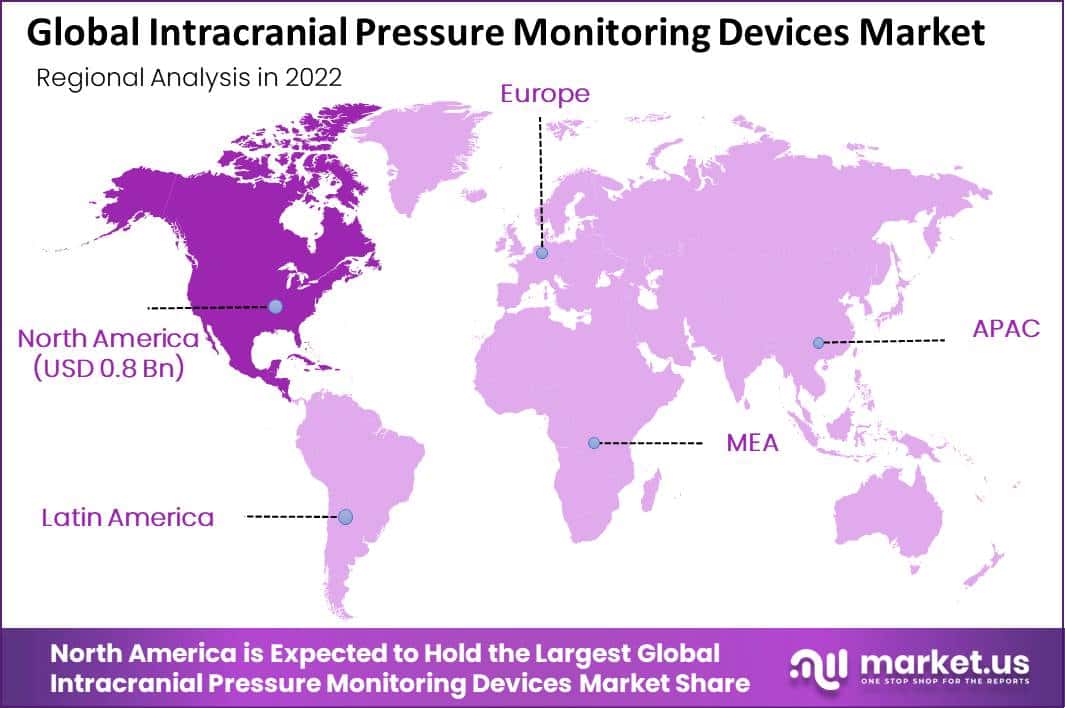
Asia Pacific is Expected to be the Fastest Growing Region in the Projected Period in the Intracranial Pressure Monitoring Devices Market.
The Asia Pacific region is expected to exhibit the highest growth rate in the intracranial pressure monitoring devices market during the forecast period. The region’s growth is attributed to factors such as the increasing incidence of traumatic brain injuries, the growing elderly population, and improving healthcare infrastructure. China and India are the largest markets in the region, with Japan, South Korea, and Australia also contributing significantly to the market’s growth.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Medtronic plc is the leading player in the market, with a significant market share. The company offers a wide range of intracranial pressure monitoring devices, including external and internal monitoring devices, and has a strong presence in several regions worldwide.
The market is also characterized by the presence of several small and medium-sized players that focus on niche product segments. These players are increasingly focusing on developing innovative products and expanding their distribution networks to gain a competitive edge in the market.
Market Key Players
- Medtronic
- Codman & Shurtleff Inc.
- RAUMEDIC AG
- Vittamed
- Sophysa
- Orsan Medical Technologies
- Integra LifeSciences
- Spiegelberg GmbH & Co. KG
- Natus Medical Incorporated
- Gaeltec Devices
- Third Eye Diagnostics
- Vivonics Inc.
- DePuy Synthes
Recent Developments
- In August 2021, Medtronic announced the launch of a new mobile application for their SynchroMed™ II intrathecal drug delivery system. The app allows patients to monitor their therapy and manage their symptoms more easily.
- In August 2021, RAUMEDIC announced the launch of its new neuromonitoring catheter, which is designed to measure intracranial pressure and temperature in real time during neurosurgery.
Report Scope
Report Features Description Market Value (2022) USD 2 Billion Forecast Revenue (2032) USD 3.8 Billion CAGR (2023-2032) 6.9% Base Year for Estimation 2022 Historic Period 2016-2021 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product (Invasive, and Non-invasive); By Application (Traumatic Brain Injury, Intracerebral Hemorrhage, Meningitis, Subarachnoid Hemorrhage, CSF Management, Migraine, Stroke, Hydrocephalus, EEG, and Other Applications); By End-User (Hospitals and Clinic, and Trauma Centers) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Medtronic, Codman & Shurtleff Inc., RAUMEDIC AG, Vittamed, Sophysa, Orsan Medical Technologies, Integra LifeSciences, Spiegelberg GmbH & Co. KG, Natus Medical Incorporated, Gaeltec Devices, Third Eye Diagnostics, Vivonics Inc., DePuy Synthes, Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the market size of intracranial pressure monitoring devices?The global intracranial pressure monitoring devices market accounted for USD 2.0 billion in 2022 and will reach USD 3.8 billion by 2032.
What do intracranial pressure monitoring devices measure?Intracranial pressure monitoring devices are medical devices designed to measure and monitor the pressure inside the skull.
Where are these intracranial pressure monitoring devices market devices typically used?These devices are typically used in critical care settings to help diagnose and manage conditions that affect the brain.
What are the driving factors for the growth of the intracranial pressure monitoring devices market?The increasing incidence of neurological disorders and advancements in technology are key driving factors for the market growth.
Which application segment holds a significant share in the market?The traumatic brain injury segment holds a significant share in the application segment of the intracranial pressure monitoring devices market.
Who are the key end-users of these devices?Hospitals and clinics are the key end-users of intracranial pressure monitoring devices, particularly in neurocritical care units.
What growth opportunities exist in the market?Emerging markets and home-based monitoring present growth opportunities for industry participants. Technological advancements and increasing demand for innovative medical technology contribute to these opportunities.
What are the latest trends in the intracranial pressure monitoring devices market?The development and use of wireless monitoring devices, non-invasive monitoring solutions, and a focus on patient safety are among the latest trends in the market.
Who are the leading players in the intracranial pressure monitoring devices market?Medtronic plc holds a significant market share and is the leading player in the market. Other players include Codman & Shurtleff Inc., RAUMEDIC AG, Vittamed, Sophysa, and more.
 Intracranial Pressure Monitoring Devices MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Intracranial Pressure Monitoring Devices MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- Medtronic
- Codman & Shurtleff Inc.
- RAUMEDIC AG
- Vittamed
- Sophysa
- Orsan Medical Technologies
- Integra LifeSciences
- Spiegelberg GmbH & Co. KG
- Natus Medical Incorporated
- Gaeltec Devices
- Third Eye Diagnostics
- Vivonics Inc.
- DePuy Synthes










