Global Human Papillomavirus (HPV) Testing Market By Type (Bivalent, Polyvalent, and Quadrivalent ), By Disease Indication (HPV Associated Cancer and Genital Warts), By Distribution Channels (Hospitals Pharmacies, Retail Pharmacies, Government Suppliers, and Others), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: Oct 2023
- Report ID: 59786
- Number of Pages: 319
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
The Global Human Papillomavirus (HPV) Testing Market size is expected to be worth around USD 13.9 Billion by 2032 from USD 4.6 Billion 2022, growing at a CAGR of 4.3% during the forecast period from 2022 to 2032.
Global human papillomavirus is a small, uncoated deoxyribonucleic acid DNA virus that can cause irregular tissue growth like warts and infects skin or mucosal cells. The human papillomavirus vaccine had a dominant market share. It is the most common sexually transmitted infection, passed through skin-to-skin contact.
Certain strains of HPV are high-risk and can cause cancers such as cervical cancer. Early discovery and medication can usually prohibit these from happening. Every year women can develop cervical cancer, oropharyngeal cancer, anal cancer, neck cancer, vaginal cancer, or precancerous lesions.
Human papillomavirus treats individuals with viral infections that have spread from one person to another by skin contact. The cervical cancer human papillomavirus vaccine segment dominates the market growth. The significant and primary factors for the growth of the human papillomavirus vaccine market are the consent of new HPV vaccines, increased initiatives by private organizations, and government initiatives for early screening and vaccination.
In most countries, public and private organizations collaborate to treat human papillomavirus infections. The low POS estimates sometimes reported in the literature include preclinical trials that are not applicable. The range of marketing costs is split constantly with suggestions on another pharmaceutical. The availability of such information may alter price negotiations for prescriptions in the drug health system. The highest cost we imagine is global marketing spending on health care providers rather than marketing spending effectiveness of products in reducing in risk of cancer and genital warts.
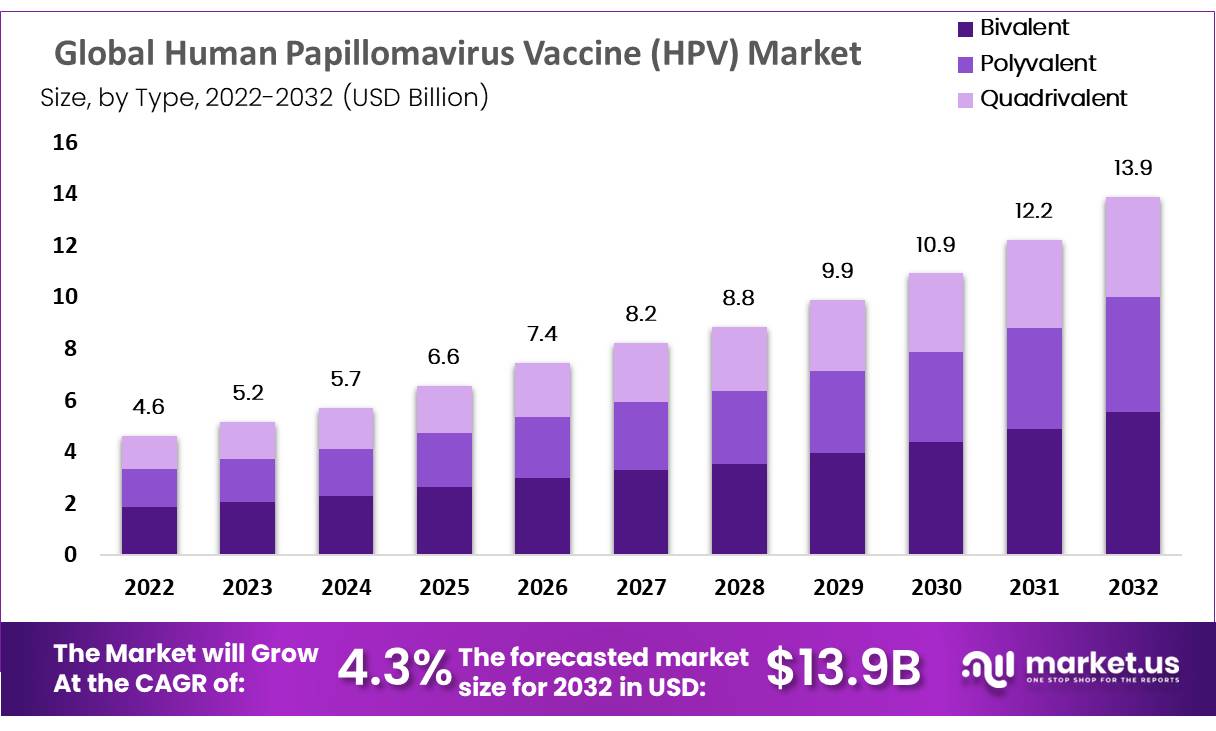
*Actual Numbers Might Vary In The Final Report
Key Takeaways
- Market Expansion: The HPV testing market experienced strong expansion due to an increasing prevalence of HPV-associated illnesses such as cervical cancer and the need for early and accurate detection.
- Primary Focus on Cervical Cancer: HPV testing was widely recognized for screening and preventing cervical cancer, providing early identification of high-risk HPV types linked to cervical cancer development. Regular screening can assist in early diagnosis.
- Testing Methods for HPV: Testing methods included nucleic acid-based techniques like Polymerase Chain Reaction and DNA hybridization assays; liquid cytology exams such as the Pap smear were often combined with HPV testing for cervical cancer screening purposes.
- Emergence of HPV Vaccination: With its increasing adoption and release, Gardasil and Cervarix vaccines were expected to have long-term impact in reducing incidences of HPV-related diseases by targeting common high-risk HPV types like HPV 16/18/19/18.
- Point-of-Care Testing Solutions: HPV testing solutions were being created specifically to allow quick and convenient HPV screening options in areas with limited resources or remote environments.
Type Analysis
Based on the type of human papillomavirus (HPV) testing market has been segmented into bivalent, polyvalent, and quadrivalent. Polyvalent vaccines, namely Gardasil/Gardasil 9 by Merck, hold the largest share in the global market. Gardasil is a quadrivalent vaccine that protects against HPV 6,11,16, and 18. Gardasil 9, a nonavalent vaccine, provides immunization against high-risk HPV strains. The vaccine benefits in the prevention of cancer and other HPV infections. As these are the only two products that protect against multiple HPV strains. They have high demand and have more sales around the world.
The higher efficiency of the polyvalent vaccine compared to the bivalent vaccine is another factor responsible for the surge in the market value of the polyvalent vaccine. Gardasil 9 is also used to treat women and men aged 27 to 45. Instead of a bivalent vaccine, GSK’s Cervarix has less demand worldwide. Cervarix is an LP1 vaccine that develops high antibodies against HPV types 16 & 18. which together cause approximately 70% of all cervical cancers. Cervarix is an essential aggressive product relative to the recently licensed L1- based preventive HPV vaccines.
Cervarix with HPV vaccine authorized for Anal Cancer produced that antibody level in women along girls was same. One study related that the group of protecting antibodies matured in adult women from Cervarix utilization and analyzed with another HPV vaccine approved for Anal Lesions and Cervical Cancer in women. The antibody levels were stable over four years. The studies considering the level of antibodies in males showed that their stories were equal to that of females.
Disease Indication Analysis
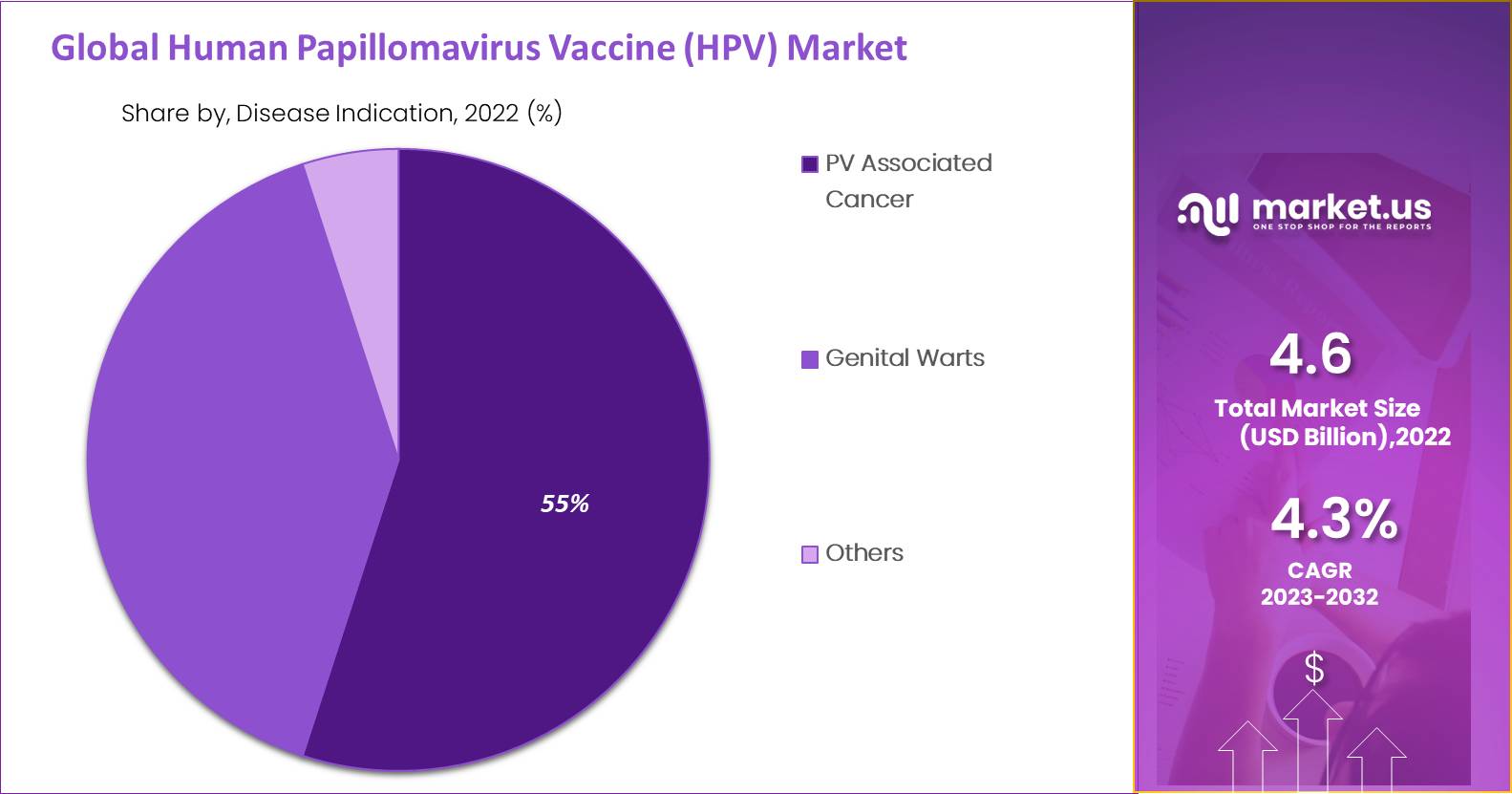
The global human papillomavirus vaccine market is segmented by disease indication analysis into HPV-associated cancer and genital warts. The dominance is attributed to higher sales of HPV vaccines globally for immunization, mostly against cervical cancer.
HPV 16 and 18 strains have been significantly identified in cervical cancer patients. The World Health Organization says cervical and anal cancer are caused due to HPV infections. The accessibility of only three vaccines has led to a more significant requirement for safety against HPV16 and HPV18-connected infections. HPV-related cancers have mostly recognized in females than males.
According to the Centers for Disease Control Organization (CDC), the prevalence rates for vulva, vaginal and cervical cancer were recorded as the highest in the forecast period. Patents often cover various stages of vaccine development and the manufacturing process. Genital warts accounted for comparatively lower market value as the infection was thought to disappear. Genital warts except severe and do not require any medical treatment; however, vaccination against genital warts is in demand.
Distribution Channel Analysis
By distribution channel, the market is further divided into hospital pharmacies, retail pharmacies, government suppliers, and others. Retail pharmacies and hospitals are expected to hold the prevailing share in the predictable period. The hospital pharmacies segment had the largest share of the market in 2022. Doctors highly prefer hospital setups for dealing with major and minor complications. Demand for HPV vaccines market is likely to exceed production capacity.
Key Market Segments
Based on Type
- Bivalent
- Polyvalent
- Quadrivalent
Based Disease Indication
- HPV Associated Cancer
- Genital Warts
Based On Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Government Suppliers
- Others
Drivers
The efficiency of HPV Vaccines to Accelerate Sales
Three clinical trials conducted by pharmaceutical companies and biopharmaceutical companies to manufacture low-cost medicines are anticipated to fuel the growth of the HPV infection vaccine market during the forecast period. The increase in the cases of HPV-associated diseases is creating awareness.
It is an effective strategy to increase the demand for HPV vaccines. One of the most significant and critical drivers of market efficiency is the protection provided by HPV vaccines to reduce adverse effects related to such infection. Bivalent and polyvalent vaccines prove their safety and efficiency through clinical trials in protecting against cancer and HPV infections. Pharmaceutical countries that improve and produce vaccines frequently face demand to lower vaccine prices to make them reasonable to developing countries.
Force analysis highlights the potency of The Medicines Patent Pool, a United Nations back-backed global health organization, and generic companies communicating with other pharmaceutical companies and international stakeholders. Limiting market power patents purchase can reduce vaccine prices and increases vaccine rates. The rise in the prevalence of HPV-related diseases is driving up the demand for HPV vaccines, which is anticipated to be the primary driving factor for the worldwide human papillomavirus (HPV) testing market opportunity during the forecast period. Major factors driving the growth of the market in the US include the well-established healthcare industry.
Increasing Efforts to Need to drive production of HPV Vaccines
However, the increase in the dominance of cervical cancer in countries worldwide is predicted to increase the sale of the human papillomavirus vaccine, thus creating market opportunity and marking the stealing market growth shortly. For instance, Gardasil sales grew from average cost during the forecast period. The immunization program by international organizations such as WHO/ ICAO/ FAO will positively contribute to HPV vaccine sales.
Restraints
Limited Products Offspring to Restrict Growth
The Human Papilloma Virus infection vaccine is not cost-effective, which may affect vaccine development and is anticipated to hinder market growth over the forecast period. Owing to the slow progress in the development of vaccines, there will be limited launches of products in the market, and limited product offerings by the manufacturers are anticipated to hamper the market growth. Comparatively inconstant immunization coverage is estimated to impact the regional size.
Opportunities
Growing Number of HPV Awareness Programs
Scientists from all over the world are researching, expanding laboratory capacity in underdeveloped countries, exchanging knowledge, and constructing global services networks to prevent and control the spread of diseases by preventive diseases. Increasing government initiatives boost market expansion during the forecast period. These factors may boost the human papillomavirus (HPV) testing market growth and opportunities.
Trends
Growing Vaccine Supply by UNICEF, WHO, and PAHO to Help Immunization Process
In the increasing prevalence of HPV-associated disorders in the GAVI-supported countries, the manufacturers are focused to rise on the production and supply of vaccine doses. International Bodies like UNICEF, WHO, and PAHO are offered to help hand by providing millions of vaccine doses to the countries in need. Thus growing the immunity population against numerous infectious diseases are the key trends in this market. PAHO and UNICEF favorably boost the adoption rate.
Regional Analysis
North America Dominates with the Largest Market Share in the Global Market
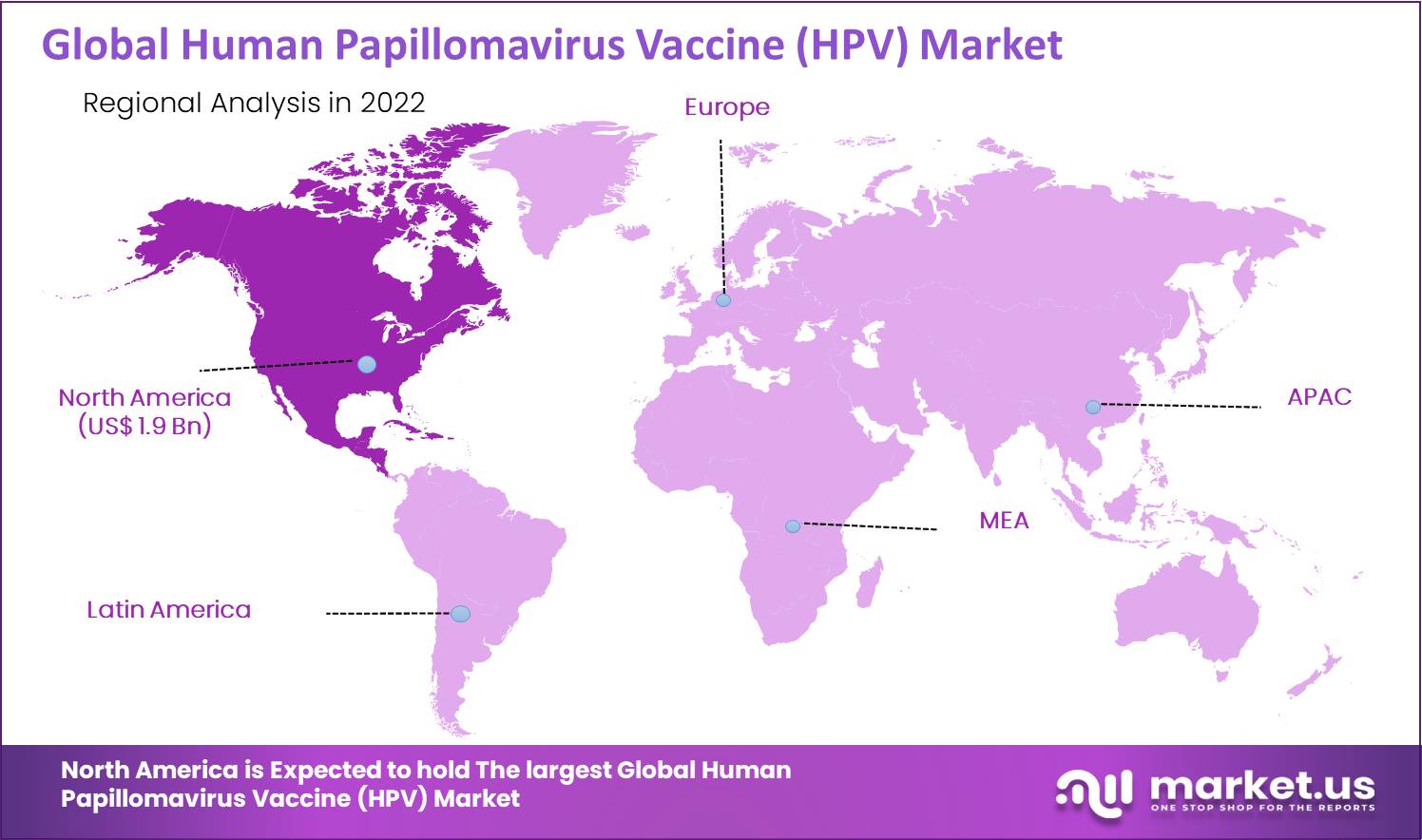
The North American region held the major revenue share with 45.0 % of the global market. During the forecast period, it is expected to dominate the global market in the coming year. Moreover, it is one of the biggest markets during the estimated period. The sales share in the US market is more in the historic period considered for future market growth than in less developed countries. Increased cases of cervical cancer and an increase in sexually transmitted diseases among the population are expected to be the primary driving factors in the market during the forecast period.
The North America region market is estimated to command the largest share of the market of global vaccines market, followed by Europe, APAC, and South America. With a large population of patients suffering from diseases, growing awareness for vaccination, and government support towards immunization, We Derive to estimate regional operating profits. Fixed costs are not typically regional pacific as manufacturing occurs in new facilities, and products are carried from there. The Asia-Pacific human papillomavirus (HPV) testing market is anticipated to grow during the forecast period.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Market players positioning facilities benchmarking provides a clear understanding of the global HPV vaccine market opportunity. The market is dominated by GlaxoSmithKline plc and Merck Co, Inc. due to their only products available for treating HPV infections.
Along with this, implementing crucial distribution strategies in developed and developing nations led to increased sales for both companies. Some leading vaccine key trends for the Human Papillomavirus Vaccine Market Players are GlaxoSmithKline PLC, Merck Co, Inc., Novartis AG, Serum Institute of India private limited, Bharat Biotech, Xenetic Biosciences Inc. are the major companies we estimate the global patent buyout price at the present discount value of the future profit.
Project launches are common strategies followed by major market players for future research, making the point that the current evident system generates extreme price-setting power. More resources are predicted to manage the sub-segment market in the expected period. However, other companies focus on launching novel vaccines against different strains of human papillomavirus. The company’s selling vaccine is Gardasil.
Merck has intense portfolio competition to GSK’S portfolio. Also, researchers are currently studying where a single dose of the HPV vaccine can be effective. Merck authorized a pediatric HIV antiretroviral medicine for the generic manufacture of LIC.
Market Key Players
- GlaxoSmithKline plc.
- Sanofi
- Pifzer Inc.
- Merck & Co. Inc.
- Novartis AG
- Emergent Biosolutions
- CSL Limited
- Xenetic Bioscienes Inc.
- Inovio Pharmaceuticals, Inc.
- Thymox Technology
- Benefit Corporation
- Bosque Solutions
- Serum Institute of India Pvt. Ltd
- Bharat Biotec.
- Other Key Players
Recent Developments
- July 2020 Merck announced that the USFDA had Accepted a prolonged indication for GARDASIL 9 to prevent oropharyngeal and other head and neck cancer caused by HPV types Human Papillomavirus Types 16,18,31, 33, 45, 52, and 58.
- June 2020 On the eve of Global Vaccine Summit 2020, vaccine manufacturers Inovax, Serum Private Institute of India Private Limited, and Walvax ramped up human papillomavirus vaccine supply availability for Gavi- Supported Countries, which have among the highest cervical cancer burdens in the world.
- January 2020 The Food and Drug Administration has raised the recommended age to receive the vaccine for human papillomavirus. Listed below are some of the most prominent Human Papillomavirus Vaccine Market industry players.
Report Scope
Report Features Description Market Value (2022) USD 4.3 Billion Forecast Revenue (2032) USD 13.0 Billion CAGR (2023-2032) 12.0% Base Year for Estimation 2022 Historic Period 2016-2021 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type – Bivalent, Polyvalent, and Quadrivalent; By Disease Indication-HPV Associated Cancer, Genital Warts; By Distribution Channel- Hospitals Pharmacies, Retail Pharmacies, and Government Suppliers Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape GlaxoSmithKline plc, Sanofi, Pfizer Inc., Novartis AG, Merck & Co., Inc. Emergent Biosolutions, Xenetic Biosciences Inc. ,CSL Limited, and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Frequently Asked Questions (FAQ)
What is the human papillomavirus (HPV) testing market growth?The global human papillomavirus (HPV) testing market is expected to grow at a compound annual growth rate of 12.0% from 2022 to 2032 to reach USD 13.0 Bn by 2032.
Which key players operating in the human papillomavirus (HPV) testing market?Some of the prominent players operating in this market place are GlaxoSmithKline plc, Sanofi, Pifzer Inc, Merck & Co.Inc, Novartis AG, Emergent Biosolutions, CSL Limited, Xenetic Bioscienes Inc, Inovio Pharmaceuticals, Inc, Thymox Technology, Benefit Corporation, Bosque Solutions, Serum Institute of India Pvt.Ltd, Bharat Biotec.
How big is the human papillomavirus (HPV) testing market?The global human papillomavirus (HPV) testing market size was estimated at USD 4.3 billion in 2022 and is expected to reach USD 4.8 billion in 2023.
 Human Papillomavirus (HPV) Testing MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample
Human Papillomavirus (HPV) Testing MarketPublished date: Oct 2023add_shopping_cartBuy Now get_appDownload Sample -
-
- GlaxoSmithKline plc.
- Sanofi
- Pifzer Inc.
- Merck & Co. Inc.
- Novartis AG
- Emergent Biosolutions
- CSL Limited
- Xenetic Bioscienes Inc.
- Inovio Pharmaceuticals, Inc.
- Thymox Technology
- Benefit Corporation
- Bosque Solutions
- Serum Institute of India Pvt. Ltd
- Bharat Biotec.
- Other Key Players










