Global Neurothrombectomy Devices Market By Product Type (Clot Retrievers, Aspiration/Suction Devices, Vascular Snares and Others), By End-User (Hospitals, Ambulatory Surgical Centers (ASCs), Emergency Clinics, and Specialty Clinics), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: Aug 2025
- Report ID: 154685
- Number of Pages: 391
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
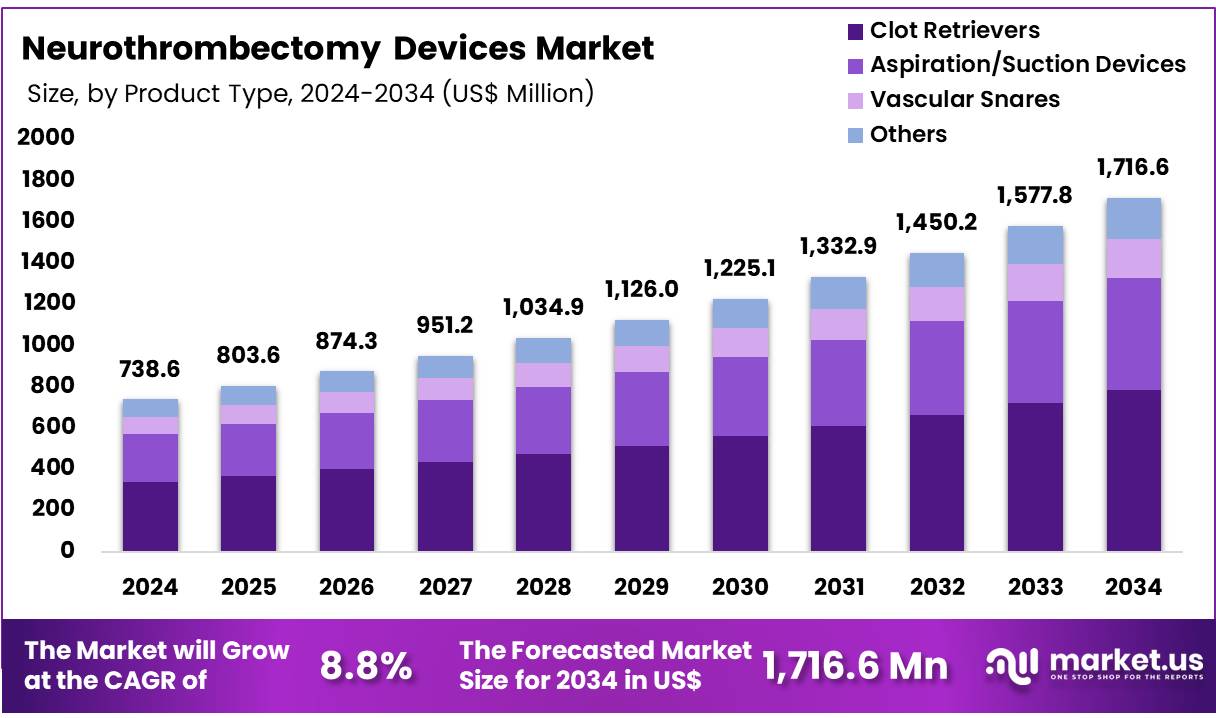
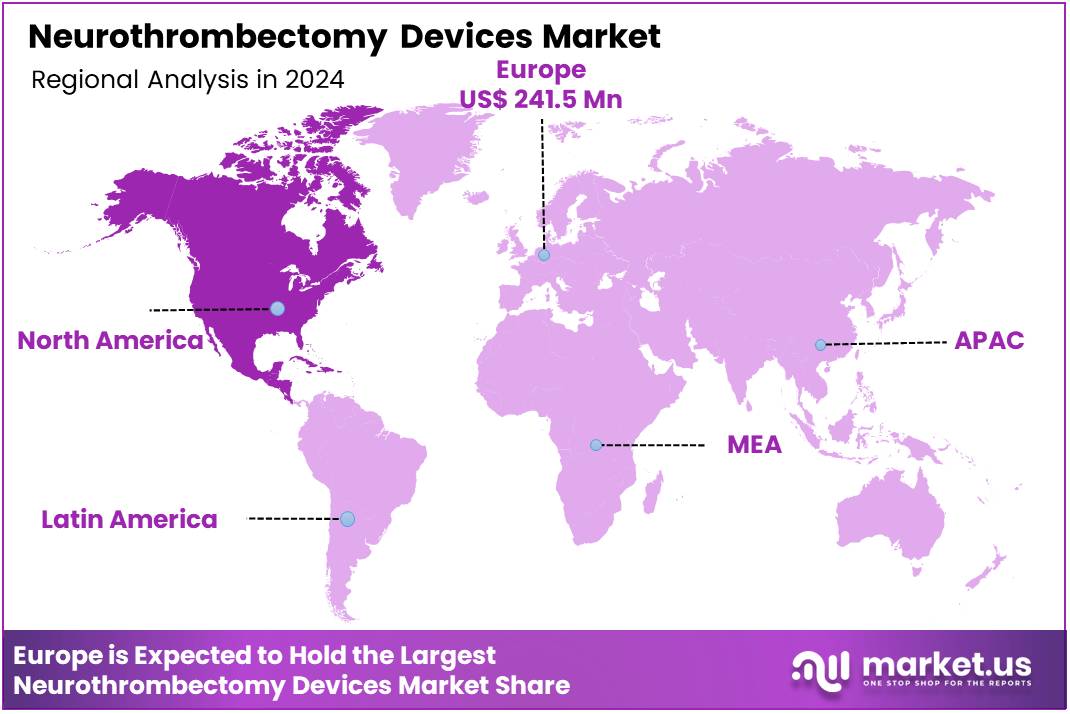
Global Neurothrombectomy Devices Market size is expected to be worth around US$ 1,716.6 Million by 2034 from US$ 738.6 Million in 2024, growing at a CAGR of 8.8% during the forecast period 2025 to 2034. In 2024, Europe led the market, achieving over 32.7% share with a revenue of US$ 241.5 Million.
Devices falling under neurohtrombectomy devices industry space are categorized into five broad classes viz; clot retrievers, aspiration/suction devices, snare-like devices, ultrasonography technologies, and lasers. Constant developments and strategic initiatives by key players in the market is a major factor which drives the market exponentially.
For instance, in March 2022, Medtronic Private Limited, a fully owned subsidiary of Medtronic plc, a global leader in healthcare technology, introduced India’s first dedicated registry for collecting real-world data on the use of revascularization devices in patients with acute ischemic stroke (AIS).
The Prospective Registry for Assessment of Acute Ischemic Stroke Patients Treated with Neurothrombectomy Devices in India (PRAAN) is a pioneering initiative designed to create a post-market registry that evaluates the clinical outcomes of Medtronic’s revascularization devices in AIS patients. This initiative puts Medtronic on the forefront of neurothrombectomy industry with overall Indian market being in the positive growth.
Below table depicts various classes and devices which have FDA indication and currently available for clinical use:
Device Class Company Name FDA Indication In Clinical Use? Aspiration/Suction Amplatz Ev3 Medical Mechanical dissolution of thrombus within dialysis fistulae No longer marketed Thrombectomy AngioJet Possis Breaking apart or removing of thrombus in peripheral veins or arterio-venous access conduits Yes NeuroJet Possis NA No longer marketed Oasis Boston Scientific Removing thrombus from hemodialysis access grafts No longer marketed Thrombectomy Penumbra Penumbra, Inc Revascularization of patients with acute ischemic stroke Yes Vasco +35 Balt Extrusion NA Not in US Clot Retriever Attractor-18 Boston Scientific NA No longer marketed Catch Balt Extrusion NA Not in US In-Time Boston Scientific Retrieval of intravascular foreign objects in peripheral vasculature, neurovasculature and cardiovasculature No longer marketed MERCI Concentric Medical Restore blood flow in the neurovasculature Yes Phenox Phenox GmbH NA Not in US TriSpan Boston Scientific NA No longer marketed Ultrasonography EKOS EKOS Corporation Infusion of fluids into peripheral vasculature Yes OmniWave OmniSonics Removal of thrombus and infusion of fluids into peripheral vasculature No longer marketed Snare Alligator Chestnut Medical Technologies, Inc Peripheral and neurovasculature foreign body removal Yes Amplatz Ev3 Medical Retrieval and manipulation of atraumatic foreign bodies in coronary and peripheral cardiovascular system and the extra-cranial neurovascular anatomy Yes Gooseneck EnSnare Device Merit Medical Systems, Inc. Retrieval and manipulation of foreign objects in the cardiovascular system or hollow viscous Yes Neuronet Boston Scientific NA No longer marketed Soutenir Solution NA Not in US Laser EPAR Endovasix Inc. NA No longer marketed LaTIS Spectranetics Removal of thrombus from vascular grafts No longer marketed Neurothrombectomy devices are used in neuro-interventional procedures to remove clots from the blood vessels (thrombus) in the brain. These devices are used majorly in the treatment of ischemic strokes in which the brain artery is blocked by a clot which results a lack of blood flow and potentially causing brain damage. According to American Heart Association data, the global rate of ischemic stroke, adjusted for age, is expected to rise to 89.32 per 100,000 people by 2030.
However, the death rate and disability-adjusted life year (DALY) rate for ischemic stroke are projected to decrease to 18.28 and 500.37 per 100,000 people, respectively. Women are expected to have a slightly higher stroke incidence than men in 2030 (90.70 versus 87.64 per 100,000). The incidence of ischemic stroke is expected to increase in all age groups and countries with different levels of development between 2020 and 2030. Among countries, Cyprus is projected to see the largest increase in stroke incidence, followed by Palestine and South Africa. Countries with lower development levels are also expected to experience a rise in death and disability rates from ischemic stroke.
The rising incidence of ischemic strokes globally is driving the market significantly. The neurothrombectomy devices are becoming as a standard treatment for acute ischemic strokes, as the demand for effective treatments to restore blood flow in blocked arteries is also escalating. The higher stroke incidence, especially among aging populations, is paving the way for new investments in advanced neurothrombectomy technologies, boosting market expansion and innovation in device efficiency and patient outcomes. This trend is expected to continue over the coming years.
For instance, in January 2023, Therma Bright Inc., known for its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other advanced medical technologies, has entered into a Share Purchase Agreement (SPA) with Inretio Ltd. to acquire its innovative blood clot retriever technology. Therma Bright has the option to invest up to US$2,000,000 in cash and US$500,000 in company shares, with the potential to earn up to a 25% stake in Inretio Inc., pending approval from the TSX Venture Exchange. The payments, made at Therma Bright’s discretion, are contingent on Inretio meeting specific milestones by May 31, 2024. The first payment of US$200,000 has already been made.
Key Takeaways
- In 2024, the market for Neurothrombectomy Devices generated a revenue of US$ 738.6 million, with a CAGR of 8.8%, and is expected to reach US$ 1,716.6 million by the year 2034.
- The product type segment is divided into Clot Retrievers, Aspiration/Suction Devices, Vascular Snares, and Others with Clot Retrievers taking the lead in 2024 with a market share of 45.7%.
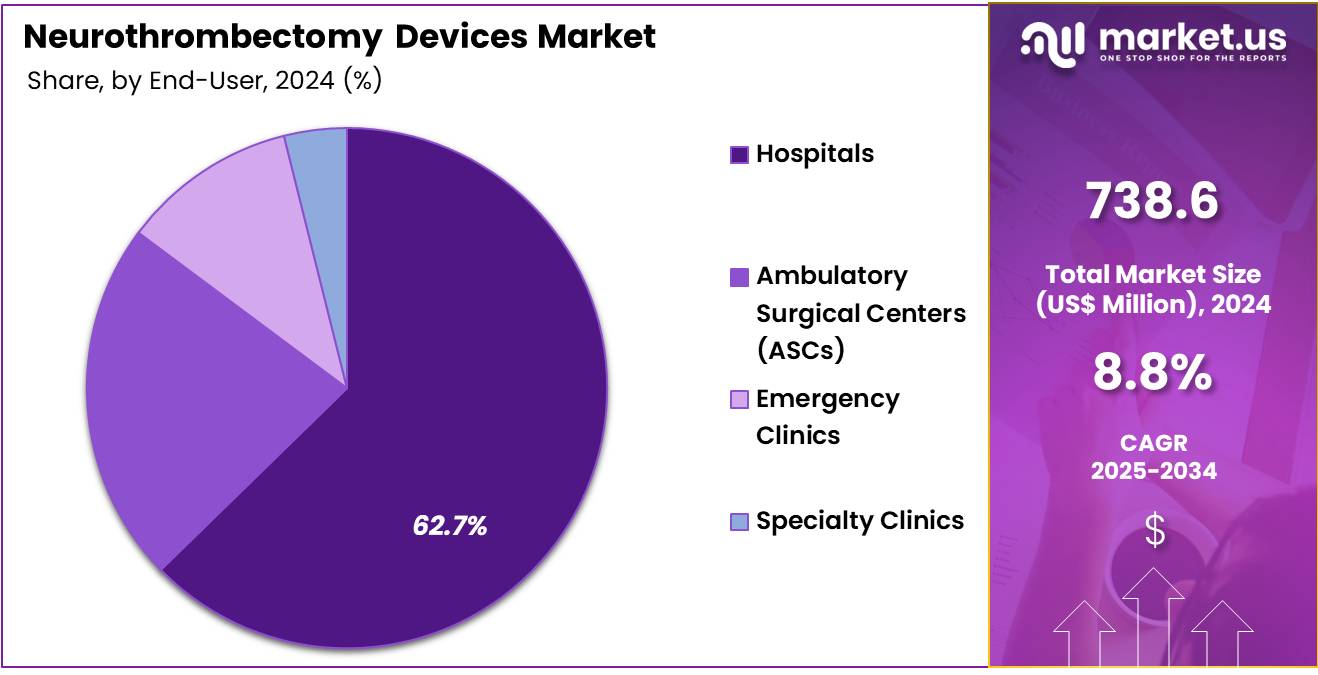
- By End-User, the market is bifurcated into Hospitals, Ambulatory Surgical Centers (ASCs), Emergency Clinics, and Specialty Clinics, with Hospitals leading the market with 62.7% of market share.
- Europe led the market by securing a market share of 32.7% in 2024.
Product Type Analysis
Clot retrievers are the most used product in the market which dominated the market in 2024 with a market share of 45.7%. Clot retrievers are very efficient in mechanically removing blood clots from cerebral arteries, hence widely used in ischemic strokes. These devices often have high demand in the market their proven success in improving patient outcomes. Companies in the market are involved in product development and securing new patents to maintain their market position and dominance.
For instance, in February 2025, Retriever Medical, a company dealing in innovative medical solutions, announced the issuance of U.S. Patent #12,201,315 B2 by the United States Patent and Trademark Office (USPTO). This patent protects a groundbreaking clot removal device, designed to revolutionize the treatment of vascular blockages. The device offers exceptional precision, adaptability, and effectiveness in eliminating unwanted material from a patient’s body, marking a significant advancement in medical technology for vascular occlusions.
End-User Analysis
Hospitals are the primary medium through which neuro-thrombectomy procedures are performed which makes hospitals a dominant segment in the market which held for over 62.7% market share in 2024. This is due to their critical role in emergency stroke interventions, where timely treatment is crucial for patient outcomes.
Hospitals play a crucial role in stroke care, offering a full range of services such as emergency care, advanced imaging technologies, and surgical interventions—all vital for neurothrombectomy procedures. With their ability to manage complex cases and provide post-procedure rehabilitation, hospitals are at the forefront of stroke management. As the primary healthcare providers, they are seeing a growing demand for advanced medical technologies, fueled by the rising incidence of strokes.
In response to this, many hospitals are starting to offer neurothrombectomy procedures to extend their service portfolio which in turn raises the demand for devices used. Governments support and extended support from organizations is also helping the market bloom. For example, in May 2025 the Technology Development Board (TDB), under the Department of Science and Technology (DST), has provided financial support to M/s S3V Vascular Technologies Limited, Mysuru, for their project titled “Integrated Manufacturing of Mechanical Thrombectomy Kit for Treatment of Acute Ischemic Stroke.
“This initiative aims to establish a cutting-edge manufacturing facility at the Medical Devices Park in Oragadam, Sriperumbudur (Chennai). The facility will focus on developing and producing advanced Mechanical Thrombectomy kits, which play a crucial role in treating acute ischemic stroke caused by large vessel occlusion, offering life-saving interventions for affected patients.
Key Market Segments
By Product Type
- Clot Retrievers
- Aspiration/Suction Devices
- Vascular Snares
- Others
By End-User
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Emergency Clinics
- Specialty Clinics
Drivers
Increasing prevalence of Acute Ischemic Strokes, especially in aging populations
Being the most common type of stroke, ischemic stroke is a significant concern on the public health and poses a burden on the same. Moreover, ischemic stroke can happen to a diverse range of populations which causes many morbidity and mortality worldwide. According to a report by U.S. Centers for Disease Control and Prevention updated in October 2024, in the United States in 2022, 1 in 6 deaths (17.5%) from cardiovascular disease was due to stroke. About 87% of all strokes are ischemic strokes
As the most common type of stroke, ischemic strokes pose a significant burden on public health systems and individuals alike. With its pervasive impact across diverse patient populations, strokes constitute a significant source of morbidity and mortality worldwide. Understanding the underlying mechanisms, risk factors, and management strategies is crucial in mitigating the impact of this pervasive neurological condition.
Neurothrombectomy devices, which help remove clots from cerebral arteries, are increasingly recognized as a critical part of stroke management. Advances in technology have made these procedures more accessible and efficient, contributing to higher adoption rates. This growing demand for life-saving interventions is expected to continue driving market expansion, particularly in developed regions.
Restraints
High procedural costs and limited access to Advanced Medical Technologies
A key challenge for the neurothrombectomy devices market is the high cost of procedures and limited access to advanced treatment options, particularly in low- and middle-income countries. These factors prevent a significant portion of the population from benefiting from life-saving neurothrombectomy procedures. The complex technology needed for these devices, coupled with the demand for highly skilled healthcare professionals, drives up the cost of treatment.
For example, according to Cost Analysis by Prudour Pvt Ltd, the total cost of hospitalization for patients undergoing mechanical (i.e., neuro-) thrombectomy for acute stroke was approximately US$24,154 (with a range reported between US$18,365 and US$29,942 for different scenarios and institutions). Similarly, the total cost associated with mechanical thrombectomy was US$148,600, compared to US$142,000 for standard medical therapy in 2024 showing that thrombectomy increases overall treatment expenditures.
Opportunities
Technological Advancements in Device efficiency and Miniaturization
These technological advancements are transforming neurothrombectomy procedures, offering clinicians more effective tools to treat acute ischemic strokes and ultimately improving patient outcomes. For example, enhanced stent retriever designs are being implemented, e.g. NeVa™ by Vesalio features a multi-segmental design with three “Drop Zones” that allow for single-pass clot capture, improving first-pass success rates and reducing procedure times.
Advanced aspiration systems are also under development which provides additional benefits and features. For instance, Penumbra JET® 7 is a neurothrombectomy device which comes with XTRA FLEX™ Technology providing improved aspiration capabilities, allowing for more effective clot removal in challenging anatomies.
Moreover, Artificial Intelligence (AI) is being employed to analyze real-time imaging data, aiding in the identification of optimal thrombectomy sites and predicting procedural outcomes. This integration supports clinicians in making informed decisions during interventions.
For instance, in May 2024, Rapid Medical, a prominent innovator in neurovascular devices, has successfully completed its first robotic thrombectomy procedure in Medellín, Colombia. The procedure involved two patients being treated with the Robotic TIGERTRIEVER, the first endovascular thrombectomy device capable of autonomously adjusting to a patient’s unique anatomy.
Impact of Macroeconomic / Geopolitical Factors
The ability of healthcare systems and individuals to afford neurothrombectomy devices is closely linked to broader economic conditions. High GDP countries (e.g., US, Germany) with substantial healthcare spending see greater market adoption, while economic downturns or limited national budgets restrict device purchases and deployment. In the US, rising healthcare expenditure going from US$11,859 in 2020 to US$12,318 in 2021 enables broader access to advanced devices and procedures.
Favorable reimbursement policies often in developed regions help drive demand by reducing out-of-pocket costs for hospitals and patients. For example, North America’s strong healthcare spending coupled with supportive reimbursement structures has helped maintain its leading market position. Surging government healthcare investments (e.g., US government spending increased 35% in 2020) accelerate adoption and market growth.
Geopolitical factors such as government regulations, trade and supply chain, the political stability of the country and government support can all impact the neurothrombectomy device market significantly. For instance, during the COVID-19 pandemic, supply chain bottlenecks and resource reallocations impacted global access to neurothrombectomy devices, disproportionately affecting low- and middle-income countries.
Recent political focus on chronic disease management in the Asia-Pacific region is a good example of this which as a result, is driving increased public and private healthcare investment, expanding access in China and India. Although the high device cost and need for specialized medical personnel remain barriers. Sanctions or trade barriers between major economies (e.g., US-China tensions) can complicate device import/export, causing localized shortages or slower technology rollout.
Latest Trends
Shift Towards Minimally Invasive Procedures
Due to non-compliance in the patient treatment for open surgeries, patients and healthcare providers are preferring minimally surgeries and opting for thrombectomy devices that offer faster recovery times, less discomfort, and fewer risks compared to traditional surgical interventions. Especially, aspiration-based thrombectomy devices are gaining more attention in the recent years.
In April 2025, Anaconda Biomed, S.L., a medical technology company focused on developing next-generation neurothrombectomy devices, announced the enrollment and treatment of the first U.S. patient in its ATHENA clinical trial. The procedure was conducted by Dr. Shahram Majidi, Associate Professor of Neurosurgery, Neurology, and Radiology at Icahn School of Medicine at Mount Sinai, New York.
ATHENA is a global, randomized pivotal study involving 327 patients, aimed at evaluating the safety and effectiveness of Anaconda’s proprietary ANA Funnel Catheter™. This device is designed to assist in stent retriever-based thrombectomy by restricting blood flow during clot retrieval, enabling simultaneous aspiration and minimizing the risk of clot fragmentation for patients with large vessel occlusion acute ischemic stroke.
Additionally, the adoption of advanced robotic assistance and real-time imaging technologies is enhancing the precision and success of these procedures. This trend is expected to continue, shaping the future of stroke care and device development in neurovascular treatments.
Regional Analysis
Europe is leading the Neurothrombectomy Devices Market
The prevalence of stroke especially AIS in Europe, particularly in the Germany, is a primary driver. Europe boasts state-of-the-art medical facilities and a high adoption rate of innovative medical technologies, facilitating the widespread use of neurothrombectomy devices. Europe held the majority of the global market share in 2024 with 32.7%, dominating the market.
Key players in the Europe neurothrombectomy devices market include Medtronic, Stryker Corporation, Penumbra Inc., and Acandis GmbH. These companies are focusing on product innovation, strategic partnerships, and expanding their market presence to capitalize on the growing demand for neurovascular interventions.
In February 2024, CERENOVUS, Inc., a part of Johnson & Johnson MedTech, announced the launch of the CEREGLIDE™ 71 Intermediate Catheter, a next-generation catheter designed with TruCourse™ technology, aimed at helping patients with acute ischemic stroke undergo revascularization. The CEREGLIDE 71 is the newest addition to the CEREGLIDE Family of Catheters within the CERENOVUS STROKE SOLUTIONS™ portfolio. It is optimized for effective direct aspiration and the delivery of compatible stent retrievers, including the EMBOTRAP™ III Revascularization Device, into the neurovasculature. This innovation enhances the efficiency and precision of stroke treatment interventions.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the Neurothrombectomy Devices market includes Medtronic Plc, Stryker Corporation, Penumbra Inc., MicroVention Inc. (Terumo Corporation), Acandis GmbH, Phenox GmbH, Vesalio LLC, Boston Scientific Corporation, Abbott Laboratories, Edwards Lifesciences Corporation, INARI Medical, LeMaitre Vascular, Inc., AngioDynamics, Inc., and Other key players.
Medtronic offers the Solitaire™ X Revascularization Device, a stent retriever designed for effective clot retrieval during thrombectomy procedures in acute ischemic stroke patients. The device features reliable clot engagement and accurate capture, with real-time visualization capabilities to enhance procedural outcomes.
Additionally, Stryker’s Trevo NXT ProVue Retriever is a next-generation stent retriever optimized for combination techniques, utilizing a responsive delivery wire for smoother navigation. Penumbra’s Neuro Thrombectomy System is a fully integrated system designed specifically for mechanical thrombectomy. It includes components like the Penumbra ENGINE® aspiration source and RED® Reperfusion Catheters, enabling effective clot removal in acute ischemic stroke patients.
Top Key Players
- Medtronic Plc
- Stryker Corporation
- Penumbra Inc.
- MicroVention Inc. (Terumo Corporation)
- Acandis GmbH
- Phenox GmbH
- Vesalio LLC
- Boston Scientific Corporation
- Abbott Laboratories
- Edwards Lifesciences Corporation
- INARI Medical
- LeMaitre Vascular, Inc.
- AngioDynamics, Inc.
- Other Prominent Players
Recent Developments
- In July 2025, Imperative Care, Inc. announced the FDA 510(k) clearance and the successful treatment of its first patients using the Zoom 7X catheter for aspiration thrombectomy in acute ischemic stroke. This device is the newest addition to the company’s comprehensive Zoom stroke system.
- In May 2024, Ceretrieve announced the successful outcomes of its multicenter, single-arm study, demonstrating the effectiveness of its advanced aspiration catheter in treating stroke. The study, conducted across two centers, involved 20 patients with acute ischemic stroke caused by intracranial large vessel occlusion (LVO), all of whom were eligible for thrombectomy within 24 hours of symptom onset. The primary goal of the study was to evaluate the safety and initial performance of the Ceretrieve device.
- In July 2023, iVascular announced that the S3MTIC study, which will include 225 patients across 20 sites in four countries: Spain (14 sites), Belgium (one site), Germany (three sites), and France (two sites) is underway, with the primary goal of assessing the safety and efficacy of treating large vessel occlusions using the company’s three neurothrombectomy devices: the iNedit balloon distal access catheter, the iNdeep microcatheter, and the iNtercept stent retriever.
Report Scope
Report Features Description Market Value (2024) US$ 738.6 Million Forecast Revenue (2034) US$ 1,716.6 Million CAGR (2025-2034) 8.8% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Clot Retrievers, Aspiration/Suction Devices, Vascular Snares and Others), By End-User (Hospitals, Ambulatory Surgical Centers (ASCs), Emergency Clinics, and Specialty Clinics) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Medtronic Plc, Stryker Corporation, Penumbra Inc., MicroVention Inc. (Terumo Corporation), Acandis GmbH, Phenox GmbH, Vesalio LLC, Boston Scientific Corporation, Abbott Laboratories, Edwards Lifesciences Corporation, INARI Medical, LeMaitre Vascular, Inc., AngioDynamics, Inc., and Other key players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Neurothrombectomy Devices MarketPublished date: Aug 2025add_shopping_cartBuy Now get_appDownload Sample
Neurothrombectomy Devices MarketPublished date: Aug 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Medtronic Plc
- Stryker Corporation
- Penumbra Inc.
- MicroVention Inc. (Terumo Corporation)
- Acandis GmbH
- Phenox GmbH
- Vesalio LLC
- Boston Scientific Corporation
- Abbott Laboratories
- Edwards Lifesciences Corporation
- INARI Medical
- LeMaitre Vascular, Inc.
- AngioDynamics, Inc.










