Glioblastoma Multiforme Treatment Market Analysis By Treatment (Chemotherapy, Immunotherapy, Targeted Therapy, Radiation Therapy, Other Treatments), By Drug Class (Temozolomide, Lomustine, Bevacizumab, Other Drug Classes), By End-use (Hospitals, Clinics, Ambulatory Surgical Centers), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Oct 2024
- Report ID: 49807
- Number of Pages: 372
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
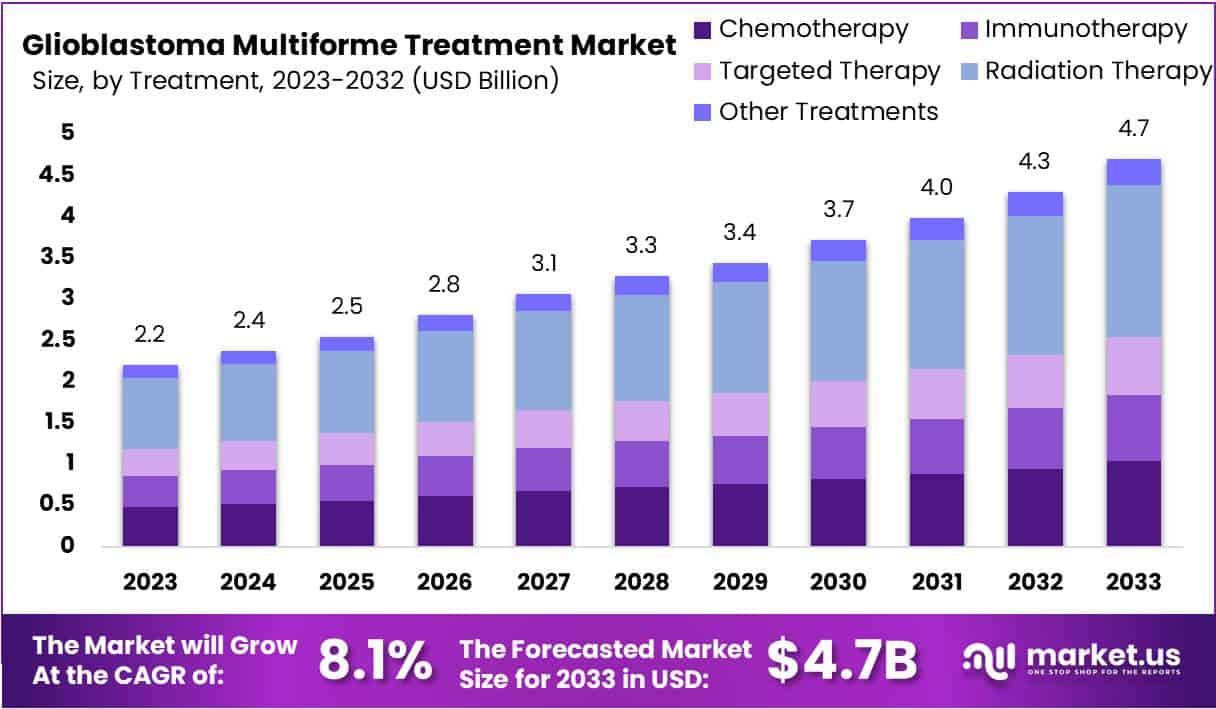
The Global Glioblastoma Multiforme Treatment Market size is expected to be worth around US$ 4.7 Billion by 2033, from US$ 2.2 Billion in 2023, growing at a CAGR of 8.1% during the forecast period from 2024 to 2033.
The glioblastoma multiforme (GBM) treatment market is intricately analyzed through various lenses. Current disease insights showcase its prevalence and impact, with a focus on advancing molecular and genetic understanding. Market dynamics are explored, encompassing size, historical trends, and growth projections, shaped by factors like incidence rates and technological strides. Treatment options, spanning surgery, radiation, and chemotherapy, are scrutinized for efficacy. The pipeline analysis unveils promising therapies in development, considering their stage, mechanisms, and potential impact.
Key industry players are identified, scrutinizing their market share, research initiatives, and recent strides. Factors driving market growth, challenges, and regulatory landscapes are assessed, alongside emergent trends like personalized medicine and immunotherapy. Patient access, affordability, and competitive landscapes are scrutinized, and investment trends in the GBM treatment sphere are tracked, creating a comprehensive overview for stakeholders and enthusiasts alike.
Key Takeaways
- The Glioblastoma Multiforme Treatment Market is anticipated to expand at an 8.1% CAGR, reaching USD 4.7 billion by 2033.
- Radiation Therapy dominates treatments, holding a 39.2% market share, known for its precision and minimal harm to healthy tissues.
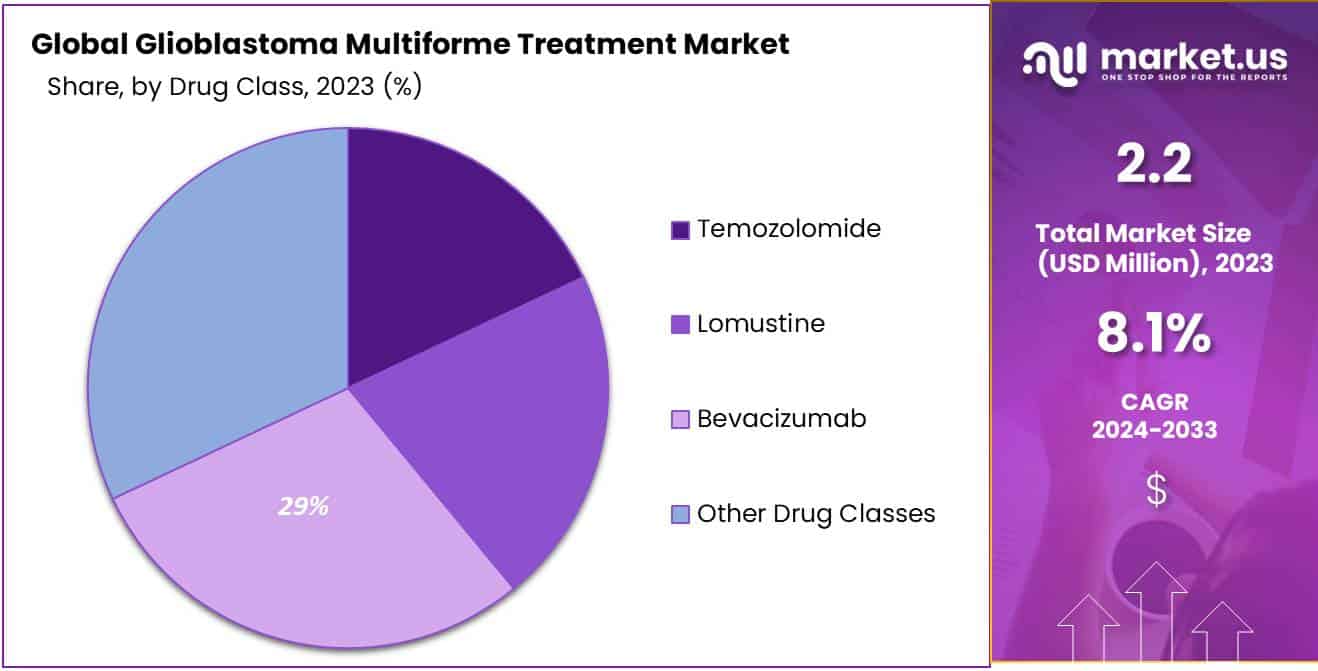
- Bevacizumab leads drug classes with a 29% market share, effectively inhibiting angiogenesis in glioblastoma multiforme treatment.
- Hospitals are the primary treatment centers, commanding over 47% of the market due to their specialized capabilities.
- Market growth is fueled by advancements in targeted therapies and immunotherapies, enhancing treatment effectiveness.
- Increasing global cases of glioblastoma multiforme are driving the need for innovative treatments.
- Collaborative research efforts are speeding up clinical trials and innovations in glioblastoma treatment.
- Government funding and initiatives are critical in supporting research and development in glioblastoma treatments.
- Challenges such as low success rates and strict regulatory approvals impede progress in effective treatments.
- Emerging trends like Precision Medicine, AI integration, and combination therapies are opening new avenues for treatment.
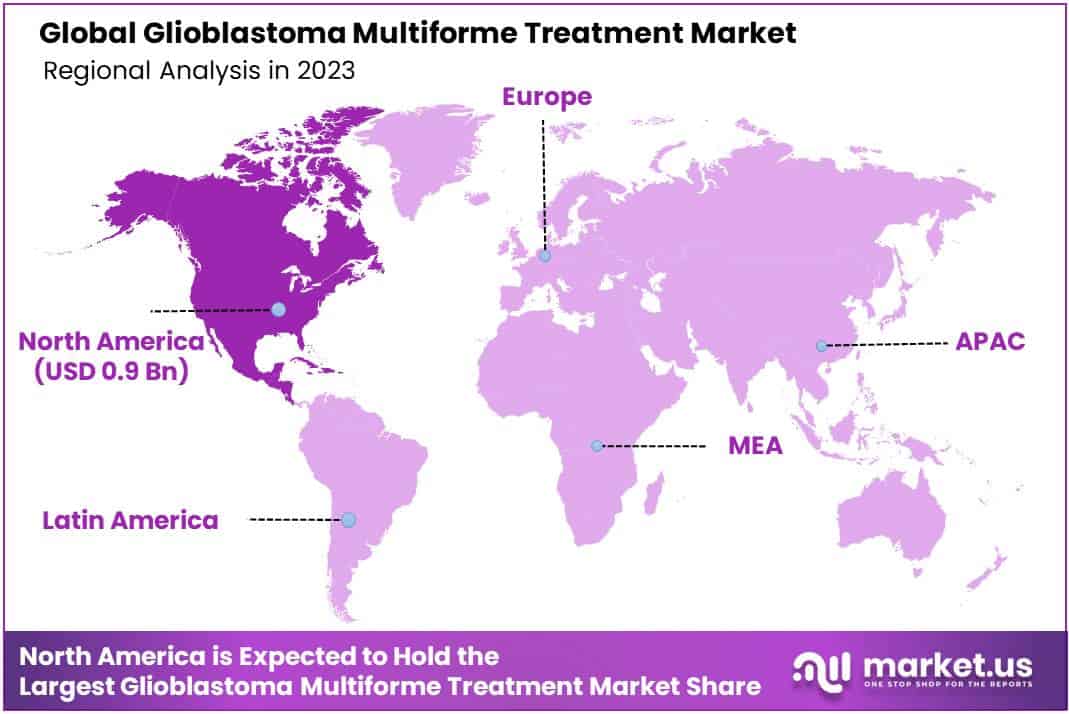
- In 2023, North America led the market with a 42.3% share, supported by advanced healthcare and research facilities.
Treatment Analysis
In 2023, the Glioblastoma Multiforme Treatment Market showcased a notable landscape, with various treatment modalities playing a crucial role in addressing this challenging medical condition. Among these, Radiation Therapy emerged as the frontrunner, securing a significant market share of over 39.2%.
Radiation Therapy, a cornerstone in glioblastoma treatment, employs high-energy rays to target and destroy cancer cells. This dominance can be attributed to its widespread acceptance and proven efficacy in managing Glioblastoma Multiforme. Patients and healthcare providers favor this approach for its ability to precisely target tumors while minimizing damage to healthy tissues.
Chemotherapy, another vital treatment avenue, also played a substantial role, contributing to the comprehensive approach against glioblastoma. The utilization of powerful drugs to impede the rapid growth of cancer cells made chemotherapy a key player in the treatment landscape, holding a considerable market share.
Immunotherapy, an innovative and promising approach, gained traction in 2023. Harnessing the body’s immune system to combat cancer, this treatment option exhibited significant potential, capturing the attention of researchers and healthcare professionals alike. Its growing market share underscored the optimism surrounding immunotherapy as a potential game-changer in glioblastoma treatment.
Targeted Therapy, marked by its precision in targeting specific molecules involved in cancer growth, also carved a niche in the market. This approach garnered attention for its potential to tailor treatment based on the unique genetic makeup of individual tumors, contributing to the diversification of treatment options available.
Furthermore, various other treatments complemented the landscape, reflecting the multifaceted nature of glioblastoma therapy. These may include supportive therapies, experimental treatments, and alternative approaches aimed at enhancing overall patient outcomes.
Drug Class Analysis
In 2023, the Glioblastoma Multiforme Treatment market witnessed a notable landscape, with distinct segments contributing to the overall market dynamics. Among the various drug classes, Bevacizumab emerged as the frontrunner, capturing a significant market share of over 29%.
Bevacizumab, a prominent player in glioblastoma treatment, demonstrated its dominance due to its effectiveness in inhibiting angiogenesis, a process crucial for the tumor’s growth. This therapeutic agent exhibited a robust 29% market share, highlighting its widespread adoption by healthcare professionals in the battle against glioblastoma multiforme.
Temozolomide, another key drug class, played a pivotal role in the market, with its efficacy in disrupting the DNA replication process of cancer cells. Lomustine, recognized for its cytotoxic properties, also made a substantial impact on the market, contributing to the comprehensive treatment strategies employed for glioblastoma.
The diverse range of drug classes underscores the multifaceted approach adopted by healthcare practitioners to combat glioblastoma. Beyond the notable dominance of Bevacizumab, each drug class plays a unique role in addressing the complexities of glioblastoma multiforme, aiming for improved patient outcomes.
End-Use Analysis
In 2023, the landscape of Glioblastoma Multiforme Treatment Market showcased a notable dominance by the Hospitals segment, securing a substantial market share exceeding 47%. Hospitals emerged as the preferred venue for glioblastoma multiforme treatment, reflecting a widespread trust among patients and healthcare providers alike.
The stronghold of Hospitals in the market is attributed to their comprehensive infrastructure, equipped with state-of-the-art medical facilities and specialized expertise. Patients seeking glioblastoma multiforme treatment often favor hospitals for their ability to provide integrated care, housing diverse medical disciplines under one roof.
Clinics, while significant players in the market, trailed behind hospitals, holding a respectable market share. These facilities offer a more streamlined and focused approach to glioblastoma multiforme treatment, catering to patients seeking specialized care in a more compact setting. Clinics are often preferred by individuals who prioritize personalized attention and a more intimate healthcare environment.
Ambulatory Surgical Centers (ASCs) have found their place in the Glioblastoma Multiforme Treatment Market, playing a role in shaping the overall dynamics of the industry. While they may have a smaller market share compared to hospitals and clinics, ASCs have gained popularity by focusing on outpatient surgical procedures. This approach resonates with patients seeking efficient and cost-effective treatment options, often avoiding prolonged hospital stays.
The varied treatment options available through Hospitals, Clinics, and Ambulatory Surgical Centers highlight the changing landscape of glioblastoma multiforme care. Each segment caters to specific patient preferences and healthcare needs, collectively contributing to the market’s growth and development in 2023. As the market continues to evolve, grasping these segmental dynamics becomes crucial for stakeholders aiming to navigate and succeed in the Glioblastoma Multiforme Treatment Market.
Key Market Segments
Treatment
- Chemotherapy
- Immunotherapy
- Targeted Therapy
- Radiation Therapy
- Other Treatments
Drug Class
- Temozolomide
- Lomustine
- Bevacizumab
- Other Drug Classes
End-use
- Hospitals
- Clinics
- Ambulatory Surgical Centers
Drivers
Advancements in Treatment Modalities
Recent breakthroughs in Glioblastoma Multiforme (GBM) treatment are transforming the landscape. Targeted therapies, immunotherapies, and combinations of treatments are taking center stage. These innovations bring a significant boost to treatment effectiveness, providing hope for improved patient outcomes.
Increasing Incidence of Glioblastoma Multiforme
The numbers are on the rise globally, signaling a pressing need for enhanced treatment options. As the prevalence of this aggressive brain tumor grows, there is a clear demand for the development of novel and more potent treatment approaches. The urgency to address this increase is driving advancements in the GBM treatment market.
Collaborative Research Initiatives
Partnerships between pharmaceutical companies, research institutions, and healthcare organizations are proving to be pivotal. These collaborative efforts speed up clinical trials, spark innovation, and push the GBM treatment market forward. The synergy from these partnerships is essential in developing and refining treatment strategies.
Government Initiatives and Funding
Governments are playing a crucial role by providing support in various forms. Grants, funding, and incentives are empowering research and development activities in GBM treatment. This financial backing acts as a catalyst, motivating pharmaceutical companies and researchers to invest in the development of innovative therapies.
Restraints
Limited Treatment Success Rates: The Uphill Battle Against Glioblastoma Multiforme
Achieving success in treating glioblastoma multiforme remains a formidable challenge due to its inherently aggressive nature. Existing treatments often fall short, offering only limited success. The relentless resistance to treatment and frequent recurrence of tumors add complexity to the pursuit of long-term remission.
Stringent Regulatory Approval Process: Navigating the Regulatory Maze
The journey to introduce new therapies for glioblastoma multiforme faces a significant roadblock—the stringent regulatory approval process. This involves rigorous clinical trials and thorough safety assessments. While essential for patient safety, these hurdles can substantially delay the timely availability of innovative treatments in the market.
High Treatment Costs: The Financial Strain on Glioblastoma Patients
Access to glioblastoma multiforme treatments, encompassing surgery, chemotherapy, and radiation therapy, is hindered by a substantial barrier high treatment costs. The financial burden associated with these interventions poses a challenge for patients, potentially limiting access to vital care. Affordability issues, particularly in regions with constrained healthcare resources, may impede market growth.
Limited Understanding of Disease Biology: Navigating the Biological Unknown
The intricate biology of glioblastoma multiforme remains a puzzle, and this incomplete understanding poses a substantial challenge. Developing targeted therapies requires a comprehensive grasp of the disease’s complexity. The current gaps in knowledge hinder the identification of optimal treatment targets, impacting the overall effectiveness of interventions.
Opportunities
Precision Medicine Revolutionizing Glioblastoma Treatment
The field of glioblastoma multiforme (GBM) treatment is witnessing a transformative shift with the increasing emphasis on precision medicine. This approach tailors treatment strategies based on individual patient characteristics, unlocking a significant growth opportunity. Personalized therapies that specifically target genetic mutations or biomarkers show promise in elevating treatment outcomes, offering patients a more tailored and effective approach to combat this challenging brain cancer.
Immunotherapy Breakthroughs
In the realm of glioblastoma multiforme treatment, there’s notable optimism stemming from the development of novel immunotherapies. Innovations like immune checkpoint inhibitors and CAR-T cell therapies hold great potential in reshaping treatment approaches. These immunotherapeutic strategies work to bolster the body’s natural immune response against tumor cells, presenting promising avenues for more effective and targeted GBM treatment.
AI’s Role in Revolutionizing Diagnosis and Treatment
Artificial Intelligence (AI) is becoming a game-changer in the landscape of GBM treatment. The integration of AI in diagnosis, treatment planning, and drug discovery processes stands as a remarkable growth opportunity. AI technologies possess the ability to analyze intricate datasets, discern patterns, and contribute to the development of more effective and precisely targeted treatment strategies for glioblastoma multiforme.
Synergistic Solutions, Exploring Combination Therapies
The horizon of glioblastoma multiforme treatment is expanding with research focused on combination therapies. The exploration of treatment modalities like chemotherapy, radiation therapy, and targeted agents in tandem offers a compelling growth opportunity. By combining these modalities strategically, there’s potential for synergistic effects that enhance treatment efficacy and address resistance mechanisms, marking a noteworthy evolution in GBM therapeutic approaches.
Trends
Liquid Biopsy Techniques: Revolutionizing GBM Monitoring for Accessibility and Real-Time Insights
A notable shift in GBM diagnostics is the rise of liquid biopsy techniques. Specifically, circulating tumor DNA (ctDNA) analysis is gaining prominence for its non-invasive nature, offering a more accessible and real-time method for monitoring glioblastoma multiforme. This trend signifies a departure from traditional invasive methods, highlighting the industry’s pursuit of more patient-friendly approaches to disease assessment.
Combination Immunotherapies: Unleashing Multifaceted Approaches to Tackle GBM’s Complex Immune Landscape
Researchers and pharmaceutical entities are delving into the exploration of combination immunotherapies for glioblastoma multiforme. Recognizing the intricate immune landscape of GBM, this trend represents a strategic response to the tumor’s complexity. The industry is acknowledging the need for multifaceted interventions, reflecting a comprehensive approach to harnessing the power of immunotherapy in GBM treatment.
Repurposing Drugs for GBM: Accelerating Development Timelines with Known Safety Profiles
A compelling trend in glioblastoma multiforme treatment involves the repurposing of existing drugs. Leveraging the established safety profiles of drugs approved for other indications, this approach accelerates the development timeline and mitigates associated risks. The increased interest in repurposing reflects a pragmatic strategy in the pursuit of effective and safe GBM treatments, showcasing a commitment to expeditious therapeutic advancements.
Regional Analysis
In 2023, North America stood out in the Glioblastoma Multiforme Treatment Market, leading with a strong market presence, claiming over 42.3% of the share. The region’s market value for the year reached an impressive USD 0.9 billion.
This remarkable dominance is attributed to advanced healthcare infrastructure and cutting-edge research initiatives. The United States played a pivotal role, boasting state-of-the-art treatment facilities and a robust healthcare system.
Factors such as increased awareness, early diagnosis, and accessibility to innovative therapies contributed to North America’s leading position. Collaborative efforts among healthcare professionals, researchers, and pharmaceutical companies further fueled advancements in glioblastoma multiforme treatment.
Moving forward, this market stronghold suggests a promising landscape for continued growth and breakthroughs in the North American region. As the journey in glioblastoma multiforme treatment unfolds, stakeholders in North America remain at the forefront, steering the market towards new horizons.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the dynamic Glioblastoma Multiforme Treatment Market, key players are actively contributing to advancements. F. Hoffmann-La Roche Ltd. stands out, leveraging extensive expertise and a commitment to research and development. As a frontrunner, they focus on innovative solutions for glioblastoma multiforme.
Other key players bring unique strengths, fostering a vibrant ecosystem where research, innovation, and patient outcomes are paramount. Collaboration among these entities ensures a multifaceted approach to tackling the challenges of glioblastoma multiforme, creating a robust environment for treatment advancements.
Market Key Players
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Merck & Co.Inc.
- Amneal Pharmaceuticals
- Arbor Pharmaceuticals LLC
- Pfizer Inc.
- Amgen Inc.
- Boston Biomedical Inc.
- Sun Pharmaceutical Industries Ltd.
- Karyopharm Therapeutics Inc.
- Other Key Players
Recent Developments
- In October 2024: Teva and mAbxience expanded their strategic partnership to include an additional oncology biosimilar candidate aimed at enhancing glioblastoma treatment options. This collaboration is part of their broader initiative to improve accessible cancer care, though specific financial details were not disclosed.
- In September 2024: Pfizer’s drug Crizotinib, currently in Phase II clinical trials for GBM, shows potential with a 23% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. Crizotinib targets cancer-specific pathways and is being evaluated for its effectiveness and approval likelihood compared to other GBM treatments.
- In June 2024: Merck has entered into a clinical study collaboration with DNAtrix to evaluate the efficacy and safety of combining DNX-2401, an oncolytic immunotherapy by DNAtrix, with Merck’s KEYTRUDA (pembrolizumab) in a Phase 2 study. This study targets patients with recurrent glioblastoma, aiming to leverage the potential synergistic effects of these therapies.
Report Scope
Report Features Description Market Value (2023) US$ 2.2 Billion Forecast Revenue (2033) US$ 4.7 Billion CAGR (2024-2033) 8.1% Base Year for Estimation 2023 Historic Period 2019-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Treatment (Chemotherapy, Immunotherapy, Targeted Therapy, Radiation Therapy, Other Treatments), By Drug Class (Temozolomide, Lomustine, Bevacizumab, Other Drug Classes), By End-use (Hospitals, Clinics, Ambulatory Surgical Centers) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape F. Hoffmann-La Roche Ltd., Teva Pharmaceutical Industries Ltd., Merck & Co.Inc., Amneal Pharmaceuticals, Arbor Pharmaceuticals LLC, Pfizer Inc., Amgen Inc., Boston Biomedical Inc., Sun Pharmaceutical Industries Ltd., Karyopharm Therapeutics Inc., Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Glioblastoma Multiforme Treatment MarketPublished date: Oct 2024add_shopping_cartBuy Now get_appDownload Sample
Glioblastoma Multiforme Treatment MarketPublished date: Oct 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Merck & Co.Inc.
- Amneal Pharmaceuticals
- Arbor Pharmaceuticals LLC
- Pfizer Inc.
- Amgen Inc.
- Boston Biomedical Inc.
- Sun Pharmaceutical Industries Ltd.
- Karyopharm Therapeutics Inc.
- Other Key Players









