Global Capnography Device Market Analysis By Component (OEM Modules (Infrared Sources, Others), Others), By Product (Hand-held, Stand-alone, Multi-parameter), By Technology (Mainstream, Sidestream, Microstream), By Application (Emergency Medicine, Pain Management, Procedural Sedation, Critical Care, Others), By End-use (Hospitals, Ambulatory Care, Others), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Jan 2024
- Report ID: 47661
- Number of Pages: 205
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
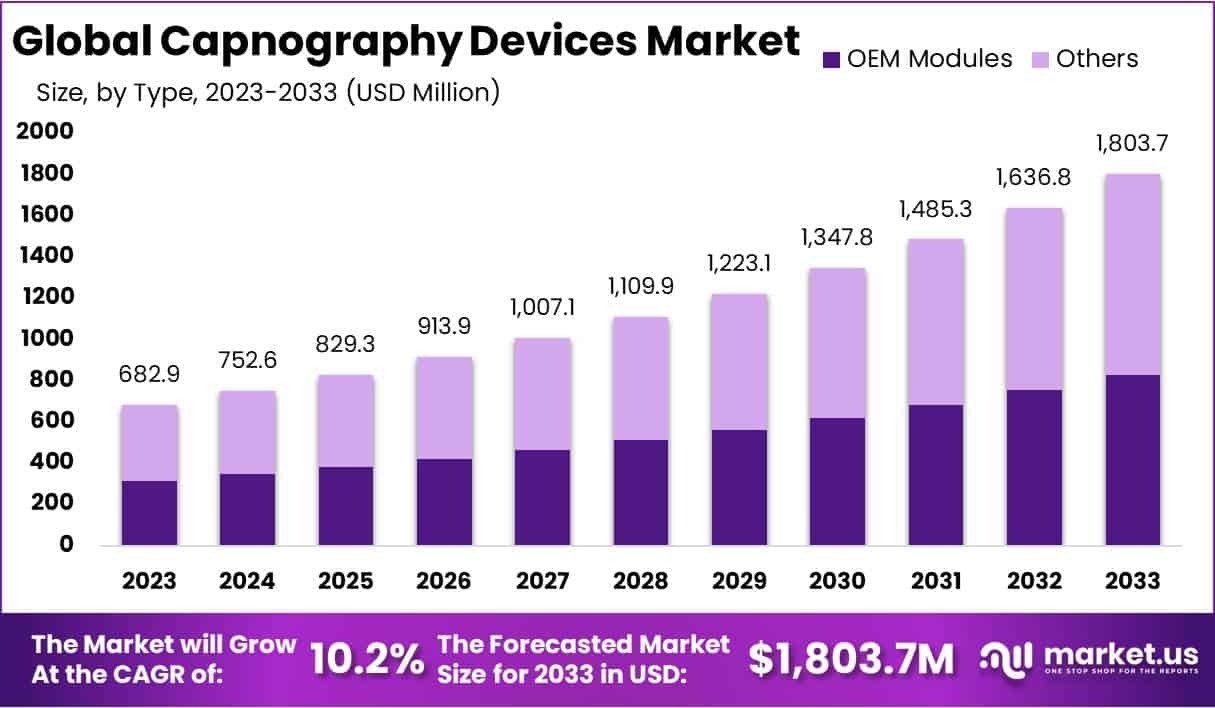
The Capnography Device Market Size is projected to reach approximately USD 1803.7 million by 2033, witnessing a significant growth from USD 682.9 million in 2023. This anticipated expansion reflects a Compound Annual Growth Rate (CAGR) of 10.2% throughout the forecast period spanning from 2024 to 2033.
Capnography devices, integral to monitoring carbon dioxide levels in exhaled breath, are undergoing a transformative evolution across diverse healthcare sectors. This comprehensive analysis delves into the current market landscape, focusing on statistics, regulatory dynamics, trade considerations, and growth catalysts shaping the trajectory of capnography devices.
Market growth is expected to be boosted by technological advances, respiratory diseases, and supportive government initiatives over the forecast period. Due to its reliability and efficiency, capnography will be more widely used in the treatment of respiratory illnesses. It is also expected to increase the efficiency of medical monitoring. According to the report by the Office of Disease Prevention and Health Promotion, asthma affects more than 25,000,000 people in the United States. Medtronic was one of the market leaders during the COVID-19 epidemic. This led to a spike in demand for capnography and other respiratory-related products.
Critical care units, operating rooms, and emergency departments are pivotal contributors to this surge, reflecting the pervasive adoption of capnography technology. As minimally invasive procedures gain prominence, ASCs are increasingly incorporating capnography for peri-operative monitoring. In the US, 85% of anesthesia care providers utilize capnography during non-emergent anesthesia cases, as reported by the American Society of Anesthesiologists.
Portable capnographs play a critical role in pre-hospital settings, enhancing rapid patient assessment and resuscitation efforts. Evidence from the Journal of Emergency Medical Services supports improved patient outcomes with the integration of capnography in EMS protocols. The utilization of home-based capnography monitoring for chronic respiratory conditions, such as COPD, is gaining momentum. The American Thoracic Society underscores its potential in managing COPD exacerbations and improving overall quality of life.
Capnographs are classified as Class II medical devices in the US, necessitating FDA clearance. The European Union’s Medical Device Regulation (MDR) imposes stringent safety and efficacy requirements for capnographs marketed in its member states. Diverse regulatory frameworks worldwide impact the import, sale, and use of capnographs, reflecting the unique compliance landscapes in each country.
Governments globally are advocating capnograph use in ambulances and home care settings, aiming to enhance patient care and mitigate healthcare costs. Initiatives also focus on raising awareness about respiratory diseases and the early detection benefits facilitated by capnography. Governments invest significantly in advanced capnograph technologies, while private companies focus on developing user-friendly and cost-effective solutions for broader market accessibility. Capnographs are increasingly integrated with other monitoring devices like pulse oximeters. The exploration of artificial intelligence (AI) aims to enhance data accuracy and interpretation, marking a notable trend in technological advancements.
Key Takeaways
- Market Size and Growth: The Capnography Device Market is projected to reach USD 1803.7 million by 2033, exhibiting a robust 10.2% CAGR from 2024 to 2033.
- Component Analysis: The Others segment dominated with a 56.5% share, including sensors, adapters, breath circuits, respirators, cuvettes, and cannulas.
- Product Analysis: In 2023, the Hand-held segment held a commanding position with a 58.3% market share.
- Technology Preference: The Sidestream segment dominated with a 52% share, showcasing its widespread use in anesthesia monitoring.
- Application Leadership: The Emergency Medicine segment demonstrated notable dominance, securing a substantial 23.4% market share.
- End-use Analysis: Hospital segment held a dominant position with a 57% revenue share due to ease-of-use, minimally invasive nature, and high adoption rates.
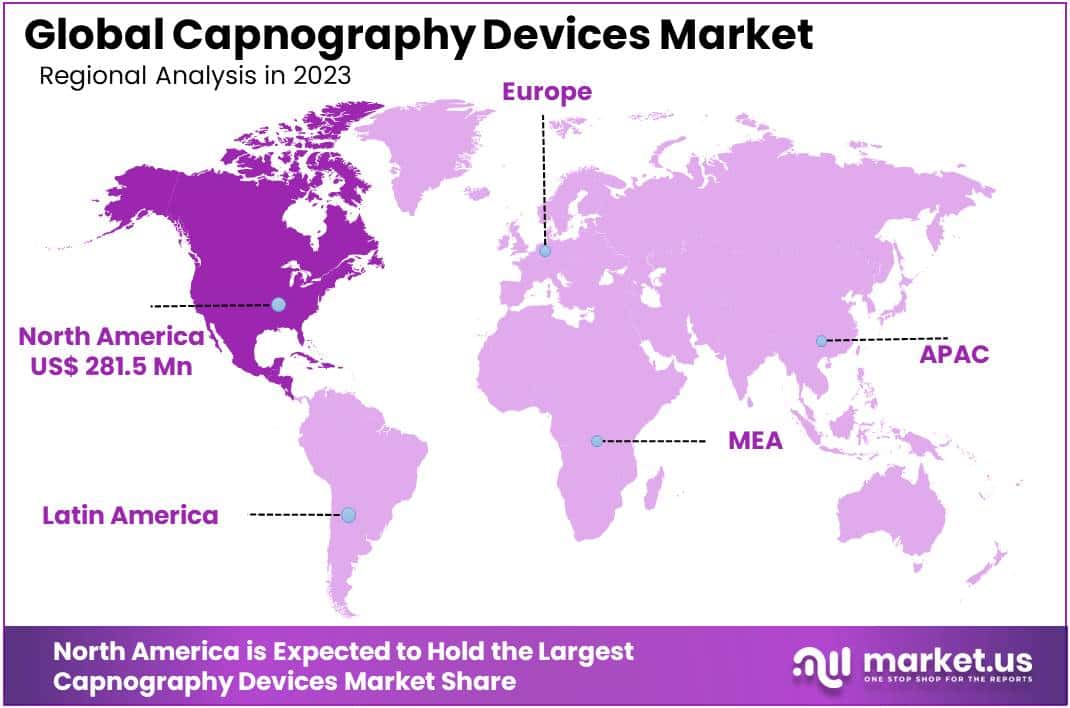
- Regional Market Share: North America led with a commanding 41.2% share, valued at USD 281.3 Million.
- Regional Growth Prospect: The Asia-Pacific region is forecasted to grow at the second-fastest CAGR, driven by improved healthcare systems.
Component Analysis
In 2023, the Others segment held a dominant market position in the Capnography Device Market, capturing more than a 56.5% share. Other components of capnography equipment include sensors & adapters, anesthetic breath circuits, anesthetic respirators, cuvettes, and cannulas. The sampling line is a key component since the accuracy of end-tidal CO2 measurements and quality waveforms are heavily dependent on it. From 2024 to 2033, the OEM modules segment is expected to experience the highest CAGR.
OEM modules’ principal function is noninvasive patient monitoring. Their adoption is fueled by this. OEM modules are also sold directly to end users who employ distributors to replace particular parts of a capnography system. This is a substantial portion. Some businesses choose to either outsource or allow third-party designers to produce OEM modules under the exact same brand name in order to satisfy the growing market demand and reduce expenses. As a result, market share has grown and total growth has accelerated. Additional categories for this segment include sources, infrared, and others.
Product Analysis
In 2023, Hand-held segment held a dominant market position, capturing more than a 58.3% share. This was due to the increasing use of handheld capnography. The benefits include easy portability, oxygen saturation levels that are required for operative procedures, long durability, and low associated cost. Hand-held Devices are now equipped with sidestream technology, an audible alarm, and a visual alarm. Hand-held Devices offer many advantages that will encourage people to prefer these instruments.
Multi-parameter products are predicted to experience the fastest CAGR over the forecast period. Multi-parameter products are expected to grow due to their growing use in emergency medicine, and procedural sedation. In capnography, advances in sensor miniaturization and the incorporation of multisensory in one device to allow simultaneous monitoring and analysis of multiple gases have allowed for faster sample collection and longer-term deployment. These factors are expected to drive demand for multiparameter instruments over the forecast period.
Technology Analysis
In 2023, Sidestream segment held a dominant market position, capturing more than a 52% share. The widespread use of sidestream technology for anesthesia monitoring explains this high growth. There are many advantages to sidestream technology-enabled medical Devices. There are many benefits, including ease of connection and fewer complications in sterilization.
This Device can also monitor substances that have not been incubated with the help of nasal adapters. Micro stream technology will experience the fastest growth over the forecast period, due to increased penetration of these products and technological advances in this field. Additionally, micro stream technology can be used for both intubated and non-intubated patients as well as patients of all ages.
Application Analysis
In 2023, the Emergency Medicine segment demonstrated notable market dominance, securing a substantial 23.4% share. Capnography Devices exhibit versatile applications, encompassing emergency medicine, pain management, procedural sedation, critical care, among others. In the realm of emergency medicine, capnography finds utility in prehospital ventilation, pediatric emergencies, and prognostication of pulmonary blood flow.
The escalating demand for capnography stems from its alignment with the guidelines and recommendations put forth by the American Heart Association (AHA) for integration into emergency medicine protocols, with a focus on enhancing survival rates. The device plays a pivotal role in procedural sedation within dentistry, showcasing a projected substantial Compound Annual Growth Rate (CAGR) over the forecast period. Its non-invasive nature allows for continuous, real-time monitoring, rendering it invaluable for assessing the patient’s metabolic and circulatory status. These inherent advantages are anticipated to propel widespread adoption, fostering significant growth in the overall market landscape in the foreseeable future.
End-use Analysis
In 2023, According to end-user, Hospital segment held a dominant market position, capturing more than a 57% revenue share. The market is divided into Ambulatory Care Centers, Hospitals, and Others. This product is used to prevent critical events in ICUs. Because of their ease-of-use, minimally invasive nature, enhanced capabilities to calculate patient ventilators measurements, and high adoption in hospital settings, capnography procedures are highly regarded. The industry is projected to see an upward trend during the forecast period.
This is due to rising rates of cardiac arrest. In turn, this will increase the need for effective patient monitoring. Additionally, organizations such as the American Society of Anesthesiologists (ASA) and the Uruguayan Society of Anesthesiologists (URSA) recommend that capnography monitoring be used during anesthesia & procedural sedation. These guidelines strong support the use of capnography Devices in hospitals. Additionally, the U.S. healthcare system has a significant influence on the global health industry which has aided the segment’s growth.
Key Market Segments
Component
- OEM Modules
- Infrared Sources
- Others
- Others
Product
- Hand-held
- Stand-alone
- Multi-parameter
Technology
- Mainstream
- Sidestream
- Microstream
Application
- Emergency Medicine
- Pain Management
- Procedural Sedation
- Critical Care
- Others
End-use
- Hospitals
- Ambulatory Care
- Others
Drivers
Increasing Awareness about Patient Safety
Rising awareness among healthcare professionals and the general public regarding the critical role of capnography in monitoring respiratory status contributes significantly to market expansion. Capnography devices, by providing real-time and continuous measurement of carbon dioxide levels during patient care, enhance early detection of respiratory complications, thereby mitigating potential risks and improving patient safety outcomes.
The increasing adoption of capnography in various healthcare settings underscores its relevance in preventing adverse events related to respiratory issues. Market research indicates a rising demand for advanced capnography devices, driven by the prioritization of patient safety, regulatory guidelines, and the pursuit of improved healthcare quality. As a result, the Capnography Device Market is poised for substantial growth in the foreseeable future.
Restraints
Cost Constraints and Budgetary Pressures
Cost constraints and budgetary pressures pose significant restraints for the Capnography Device Market. In the realm of market research, these factors impact the adoption and growth of capnography devices. Healthcare facilities and providers often face budget limitations, hindering their capacity to invest in advanced monitoring technologies like capnography. The cost of acquiring, implementing, and maintaining these devices can be a decisive factor for smaller clinics or resource-constrained settings.
Additionally, reimbursement challenges further amplify the financial burden on healthcare organizations. As a result, the market may experience slower expansion and adoption rates, especially in regions where economic considerations play a pivotal role in healthcare purchasing decisions. Successful market strategies must address these cost-related challenges to promote wider accessibility and market penetration for capnography devices.
Opportunities
Technological Advancements and Product Innovations
The Capnography Device Market stands to benefit significantly from ongoing technological advancements and product innovations. As cutting-edge technologies continue to emerge, such as miniaturization, wireless connectivity, and advanced sensors, they pave the way for more efficient and user-friendly capnography devices. These innovations enhance the accuracy, portability, and data accessibility of capnography devices, meeting the evolving demands of healthcare professionals.
Moreover, the integration of smart features and real-time monitoring capabilities further amplifies the market’s potential. Market research indicates that the adoption of these advanced technologies can lead to increased market share to improve patient outcomes and streamline medical processes. As a result, the Capnography Device Market is poised to capitalize on these opportunities by aligning with technological trends and delivering innovative products that cater to the evolving needs of the healthcare industry.
Trends
Increasing Integration of Capnography in Anesthesia and Critical Care
The Capnography Device Market is witnessing a significant trend with the increasing integration of capnography in anesthesia and critical care settings. Capnography, a non-invasive method of monitoring carbon dioxide levels in exhaled breath, is becoming indispensable in ensuring patient safety and optimizing respiratory management. The technology provides real-time, continuous feedback on a patient’s ventilation, aiding anesthesiologists and critical care professionals in timely intervention and decision-making.
This trend is driven by the growing awareness of the clinical benefits of capnography, including early detection of respiratory complications and improved patient outcomes. As healthcare providers recognize the value of capnography in enhancing patient care, the demand for advanced and integrated capnography devices is on the rise. The market is responding with innovative solutions that offer seamless integration into anesthesia and critical care workflows, contributing to the overall growth of the Capnography Device Market.
Regional Analysis
In 2023, North America dominated the Capnography Device Market, securing a commanding 41.2% share and a market value of USD 281.3 Million. This leadership is attributed to a stringent regulatory environment, particularly in the United States, fostering the adoption of cutting-edge medical technologies. The region’s well-established healthcare infrastructure, coupled with a focus on patient safety, has propelled the demand for capnography devices.
Rising awareness among healthcare professionals and the prevalence of respiratory diseases have further fueled this growth. North America’s robust economic landscape and substantial healthcare expenditure have facilitated widespread adoption of advanced medical devices. Key market players’ strategic initiatives and collaborations have also contributed to North America’s market dominance. While North America took the lead in 2023, the Asia-Pacific region shows promise with a burgeoning healthcare infrastructure, increased investments, and growing awareness, signaling potential shifts in the global capnography device market dynamics.
Based on geography, the global market can be divided into North America, Asia Pacific, South America, Europe, and the Middle East & Africa. The Asia Pacific regional market is forecast to grow at a second fastest CAGR. This high growth can be attributed to improvements in healthcare systems and an increase in healthcare expenditure. It also reflects the growing awareness of end users in the region regarding the adoption of capnography technology.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global capnography device market is witnessing substantial growth, driven by the increasing awareness of respiratory monitoring and the rising prevalence of respiratory diseases. Key players in this market play a crucial role in shaping industry trends, driving innovation, and meeting the growing demand for advanced capnography devices. This analysis delves into the profiles of key players, highlighting their market presence, product portfolios, strategic initiatives, and future outlook.
The capnography device market is characterized by intense competition and a focus on technological advancements. Key players are committed to enhancing patient outcomes, collaborating with healthcare professionals, and expanding their global footprint through strategic initiatives. As the demand for respiratory monitoring solutions continues to rise, these key players are poised to shape the future landscape of the capnography device market.
Market Key Players
- Masimo
- Smiths Medical
- Welch Allyn
- Koninklijke Philips N.V.
- Medtronic
- Nonin Medical Inc.
- BD
- Diamedica Ltd.
- Edan Instruments Inc.
- ZOLL Medical
- Edan Instruments Inc.
- GE Healthcare
- Other Key Players
Recent Developments
- In January 2024, BD disclosed its acquisition of CFlow Medical, a specialized firm focusing on innovative CO2 monitoring technology for minimally invasive procedures. This strategic acquisition serves to fortify BD’s comprehensive portfolio within the realm of respiratory and critical care solutions, aligning with the company’s commitment to advancing healthcare technologies.
- In January 2024, ZOLL Medical entered into a strategic partnership with Masimo, aimed at integrating Masimo’s SET Pulse Oximetry technology into ZOLL’s ResQ System. This collaboration is envisioned to elevate patient monitoring standards within the domain of emergency medical services, underscoring the significance of technological synergies in advancing healthcare solutions.
- In December 2023, Koninklijke Philips N.V. achieved FDA clearance for the Philips Respironics Canopy SideStream Capnography module, thereby expanding the company’s capnography capabilities specifically tailored for critical care ventilators. This regulatory milestone underscores Philips’ commitment to enhancing its offerings in the critical care segment.
- In October 2023, Masimo unveiled the Rad-G Pulse CO2 Capnometer, a compact and portable device strategically engineered for the purpose of conducting spot-checks on end-tidal CO2 levels among non-intubated patients. This product launch represents a notable advancement in the landscape of respiratory monitoring solutions.
Report Scope
Report Features Description Market Value (2023) USD 682.9 Mn Forecast Revenue (2033) USD 1803.7 Mn CAGR (2024-2033) 10.2% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Component (OEM Modules (Infrared Sources, Others), Others), By Product (Hand-held, Stand-alone, Multi-parameter), By Technology (Mainstream, Sidestream, Microstream), By Application (Emergency Medicine, Pain Management, Procedural Sedation, Critical Care, Others), By End-use (Hospitals, Ambulatory Care, Others) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Masimo, Smiths Medical , Welch Allyn, Koninklijke Philips N.V., Medtronic, Nonin Medical Inc., BD, Diamedica Ltd., Edan Instruments Inc., ZOLL Medical, Edan Instruments Inc., GE Healthcare, and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the size of the Capnography Device market in 2023?The Capnography Device market size is USD 682.9 million in 2023.
What is the projected CAGR at which the Capnography Device market is expected to grow at?The Capnography Device market is expected to grow at a CAGR of 10.2% (2024-2033).
List the segments encompassed in this report on the Capnography Device market?Market.US has segmented the Capnography Device market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Component the market has been segmented into OEM Modules (Infrared Sources, Others), Others. By Product the market has been segmented into Hand-held, Stand-alone, Multi-parameter. By Technology the market has been segmented into Mainstream, Sidestream, Microstream), By Application (Emergency Medicine, Pain Management, Procedural Sedation, Critical Care, Others. By End-use the market has been segmented into Hospitals, Ambulatory Care, Others.
List the key industry players of the Capnography Device market?Masimo, Smiths Medical , Welch Allyn, Koninklijke Philips N.V., Medtronic, Nonin Medical Inc., BD, Diamedica Ltd., Edan Instruments Inc., ZOLL Medical, Edan Instruments Inc., GE Healthcare, and Other Key Players
Which region is more appealing for vendors employed in the Capnography Device market?North America is expected to account for the highest revenue share of 41.2% and boasting an impressive market value of USD 281.3 million. Therefore, the Capnography Device industry in North America is expected to garner significant business opportunities over the forecast period.
Name the key areas of business for Capnography Device?The US, Canada, India, China, UK, Japan, & Germany are key areas of operation for the Capnography Device Market.

-
-
- Masimo
- Smiths Medical
- Welch Allyn
- Koninklijke Philips N.V.
- Medtronic
- Nonin Medical Inc.
- BD
- Diamedica Ltd.
- Edan Instruments Inc.
- ZOLL Medical
- Edan Instruments Inc.
- GE Healthcare
- Other Key Players










