APAC Transcatheter Aortic-Valve Replacement Market Analysis By Product Type (Balloon-expandable TAVR devices, Mechanically-expandable TAVR devices, Self-expanding TAVR devices), By Procedure Type (Transfemoral Implantation, Transapical Implantation, Transaortic Implantation), By End-Use (Hospitals, Specialty Clinics, Ambulatory Surgical Centers, Research/ Academic institutes), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: Apr 2024
- Report ID: 82564
- Number of Pages: 396
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
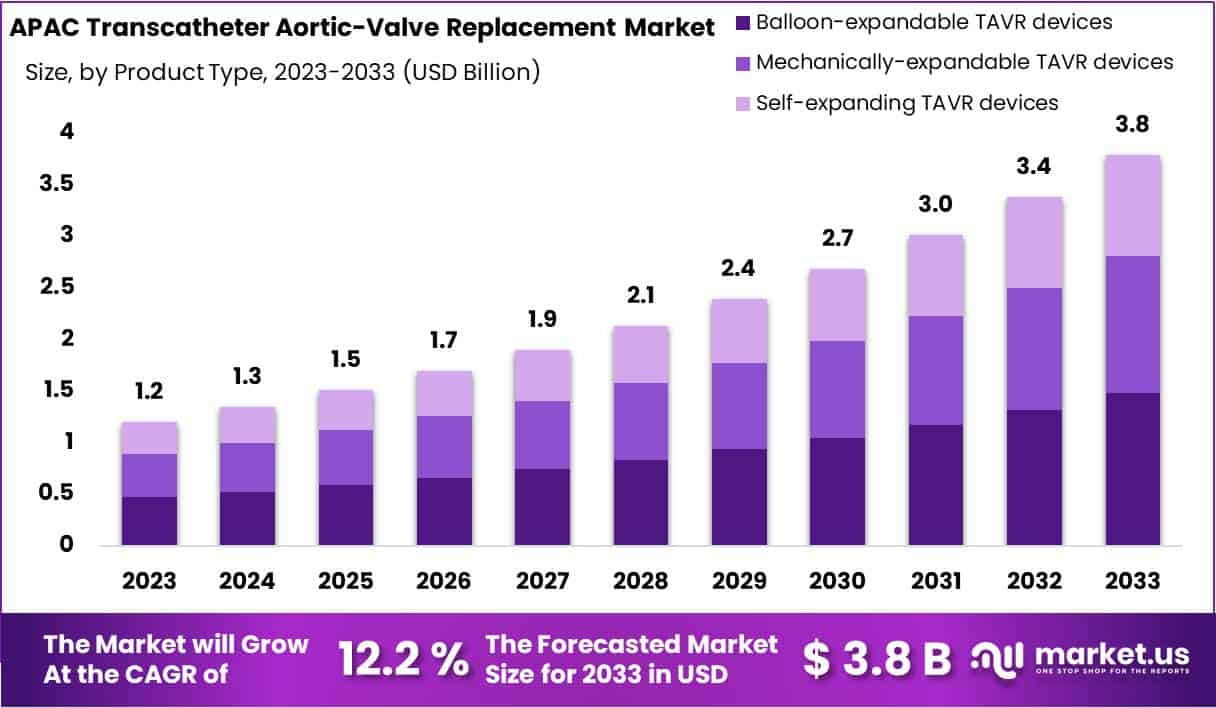
The APAC Transcatheter Aortic-Valve Replacement Market size is expected to be worth around USD 3.8 Billion by 2033, from USD 1.2 Billion in 2023, growing at a CAGR of 12.2% during the forecast period from 2024 to 2033.
Transcatheter Aortic Valve Replacement (TAVR), known within the Asia-Pacific (APAC) region, pertains to a minimally invasive surgical procedure aimed at replacing a narrowed aortic valve that fails to open properly (aortic valve stenosis). This innovative treatment approach offers an alternative to the more invasive open-heart surgery, specifically targeting patients at intermediate, high, or prohibitive risk for conventional surgery.
The APAC TAVR market is experiencing significant growth, driven by the rising prevalence of aortic valve stenosis, aging populations, and advancements in healthcare infrastructure across the region. This market expansion is further supported by increasing medical expertise and the availability of technologically advanced devices. Factors such as government initiatives aimed at improving healthcare access and the rising demand for minimally invasive procedures are contributing to the positive trajectory of the APAC TAVR market. The continued development and adoption of TAVR in the APAC region underscore its potential to enhance patient outcomes and offer substantial market opportunities for industry stakeholders.
Demographic shifts towards an older population in the APAC region contribute to a higher incidence of cardiovascular conditions, driving the need for TAVR procedures. The procedure’s minimally invasive nature aligns with the increasing patient and healthcare provider preference over more invasive methods. According to the American College of Cardiology, TAVR’s utilization has dramatically increased across all age groups, even among younger patients, indicating broadening acceptance and adoption of the procedure for treating severe aortic stenosis.
TAVR’s success is underscored by its efficacy and the rapid recovery it enables. Most TAVR procedures can be performed in a minimally invasive manner, allowing patients to be mobile the same day and typically go home the following day. This advantage, along with the potential for TAVR to treat patients over 65 with suitable anatomy effectively, signifies a major shift in treatment paradigms for aortic stenosis.
Given these dynamics, market stakeholders are advised to closely monitor demographic trends and healthcare policy developments in the APAC region. The growth trajectory for TAVR is particularly promising here, fueled by the rising prevalence of valvular heart diseases and an increasing aging population susceptible to cardiovascular diseases, including aortic valve disorders. The region is also witnessing growing investments in healthcare infrastructure, including cardiac care facilities and specialized centers for cardiovascular interventions like TAVR, which are expected to further drive market growth.
Key Takeaways
- Market expected worth USD 3.8 billion by 2033, growing at 12.2% CAGR from 2024 to 2033.
- Rising prevalence of aortic valve stenosis and aging population drive market growth in APAC region.
- Balloon-expandable TAVR devices lead market, followed by Mechanically-expandable and Self-expanding devices.
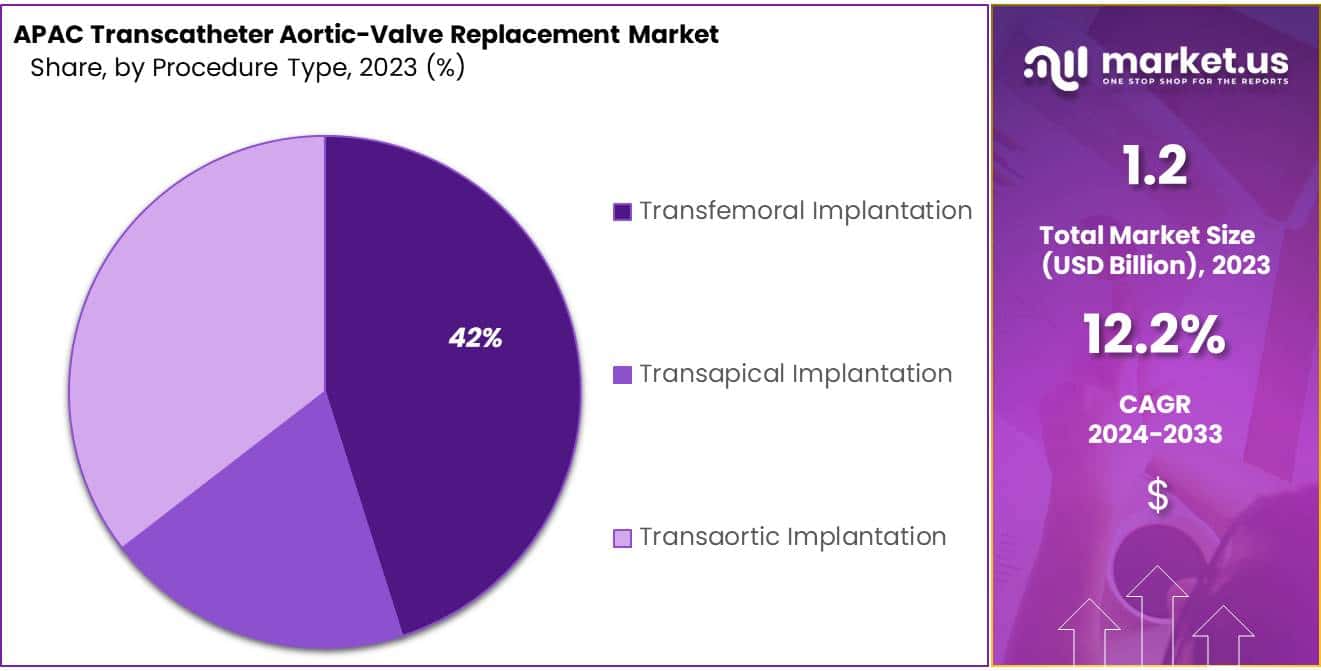
- Transfemoral Implantation procedure dominates with over 42% market share in 2023.
- Hospitals hold over 42% market share, followed by Specialty Clinics and Ambulatory Surgical Centers.
- Increasing healthcare access, expanding insurance coverage drive market growth in APAC region.
- Regulatory hurdles and cost constraints challenge market expansion in APAC countries.
- Integration of AI in diagnostics presents transformative trend, enhancing patient selection and procedural outcomes.
Product Type
In 2023, the segment for Balloon-expandable TAVR devices took the lead in the APAC Transcatheter Aortic Valve Replacement Market, boasting a share exceeding 39%. This commanding presence stems from the device’s benefits, such as enhanced control over placement and efficacy for patients with vascular issues. The rising cases of aortic stenosis among the region’s elderly population and the shift towards minimally invasive procedures have further fueled demand. Additionally, the Mechanically-expandable TAVR devices have carved out a substantial market share, attributed to their flexibility and the ability for post-deployment adjustment, appealing to a wide patient base and clinical needs.
On the other hand, the Self-expanding TAVR devices segment is witnessing steady growth, driven by their design that ensures a snug fit and minimizes leakage risks. The use of nitinol for self-expansion offers secure valve placement, with recent innovations enhancing device navigation and placement in complex cases. The overall APAC market is on an upward trajectory, bolstered by increased healthcare spending, greater procedural awareness, and advancements in healthcare infrastructure. The continuous rise in cardiovascular disease prevalence across the region underscores the growing demand for innovative treatment options like TAVR, setting the stage for sustained market growth.
Procedure Type
In 2023, the Asia-Pacific market for Transcatheter Aortic Valve Replacement (TAVR) saw the Transfemoral Implantation procedure leading in popularity, claiming over 42% of the market share in the Procedure Type Segment. This method’s ascendancy is largely due to its minimal invasiveness, gaining favor among medical professionals and patients alike, especially given the growing incidence of aortic stenosis within the region’s aging populace. Additionally, the Transfemoral approach is associated with fewer complications and a reduced hospitalization period when contrasted with other surgical alternatives, enhancing its appeal.
On the other hand, the Transapical Implantation technique also secured a notable market share, serving as an essential option for patients with conditions that render the Transfemoral approach unsuitable. Meanwhile, the Transaortic Implantation method, although utilized less frequently, is slowly emerging as a preferred choice for individuals ineligible for the former procedures due to specific health or anatomical issues. The ongoing advancements in medical technology, coupled with increasing healthcare accessibility, promise a bright future for the APAC TAVR market. This diversity in procedural approaches underscores the industry’s dedication to catering to a wide array of patient needs, ensuring broader treatment availability for those suffering from aortic stenosis.
End-Use
In 2023, the APAC Transcatheter Aortic-Valve Replacement (TAVR) Market saw the Hospitals segment leading the charge, securing over 42% of the market share. This dominance is largely due to hospitals’ comprehensive facilities for cardiac care and advanced medical technologies, which are crucial for TAVR procedures. Patients favor hospitals for these complex interventions, benefiting from specialized healthcare teams and robust post-operative support. Additionally, Specialty Clinics have carved out a significant niche, propelled by their focus on cardiac care and the personalized treatment they offer. These clinics have gained traction by catering to the growing demand for specialized cardiac services, marking their prominence in the market landscape.
On another front, Ambulatory Surgical Centers (ASCs) have made notable strides by offering cost-effective TAVR solutions. Their appeal lies in the promise of reduced hospital stays and a quicker recovery process, aligning with the shift towards outpatient care models that prioritize patient convenience and cost savings. Meanwhile, Research and Academic Institutes, despite holding a smaller market share, are instrumental in the TAVR field’s evolution. Their relentless pursuit of innovation, through ongoing research and clinical trials, is vital for advancing TAVR techniques and technologies. This collective effort from various segments underscores the dynamic nature of the APAC TAVR market, setting the stage for its continued growth and the refinement of treatment methodologies.
Key Market Segments
Product Type
- Balloon-expandable TAVR devices
- Mechanically-expandable TAVR devices
- Self-expanding TAVR devices
Procedure Type
- Transfemoral Implantation
- Transapical Implantation
- Transaortic Implantation
End-Use
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Research/ Academic institutes
Drivers
Increasing Healthcare Access
The expansion of healthcare infrastructure and accessibility, especially in rural and underserved regions of Asia-Pacific (APAC) countries, is a significant driver for the transcatheter aortic-valve replacement (TAVR) market. This trend is primarily fueled by substantial investments in healthcare IT and digital health innovations over the past decade, aiming to enhance healthcare delivery systems and increase healthcare expenditure across the region. The COVID-19 pandemic has notably accelerated the adoption of digital healthcare, including telemedicine, which nearly doubled in usage among both consumers and physicians in countries like China and Indonesia. The heightened focus on digital health solutions reflects a broader commitment to improving access to advanced medical treatments, including TAVR procedures, through enhanced healthcare infrastructure.
Restraints
Regulatory Hurdles
Regulatory hurdles significantly restrain the transcatheter aortic valve replacement (TAVR) market in the Asia-Pacific (APAC) region, primarily due to the diverse and stringent regulatory frameworks that vary across countries. These complexities not only delay the approval and adoption of TAVR devices but also pose significant challenges for manufacturers and healthcare providers aiming to navigate these regulations efficiently.
For instance, in India, TAVR procedures are performed at around 30 centers, which falls short of the demand for cardiac surgeries. This limitation is partly due to the lack of availability of skilled healthcare professionals and the complexities of the approval systems by regulatory bodies, alongside the absence of specialized training programs.
Moreover, the TAVR market, which includes the APAC region, is driven by the aging population and the rising prevalence of cardiovascular diseases. With an increasing geriatric population susceptible to aortic stenosis (AS), the demand for TAVR is expected to rise. Studies have shown that the prevalence of AS increases significantly with age, affecting 9.8% of those aged 80-89 years.
Opportunities
Expanding Insurance Coverage
Expanding insurance coverage in the Asia Pacific (APAC) region is emerging as a pivotal factor, catalyzing the growth of the Transcatheter Aortic Valve Replacement (TAVR) market. This growing prevalence of cardiovascular disorders and an aging population highly susceptible to such conditions. Japan, with its significant elderly population, is anticipated to lead the market, having accounted for over 60% of the TAVR procedures in the APAC region.
Moreover, the gradual inclusion of TAVR procedures in both national and private insurance schemes across the APAC region is notably reducing financial barriers for patients. This insurance expansion is not only making TAVR procedures more accessible but is also addressing the high costs associated with such medical interventions, which previously acted as a significant deterrent to their adoption. For instance, Japan’s government insurance coverage for TAVR procedures underlines the role of supportive policies in fostering market growth.
Trends
Integration of AI in Diagnostic Procedures
The integration of Artificial Intelligence (AI) in diagnostic procedures is emerging as a transformative trend in the Asia-Pacific Transcatheter Aortic-Valve Replacement (TAVR) market. This trend is underpinned by significant investments in healthcare IT infrastructure and technological advancements in machine learning algorithms, which have enabled the deployment of AI-powered systems in diagnostics. These systems are increasingly adopted for their ability to provide efficient and accurate diagnoses, which are crucial for optimizing patient selection for TAVR procedures, thereby improving outcomes and minimizing procedural risks .
Regional Analysis
In 2023, the APAC region boasts a notable market value of USD 3.8 billion in Transcatheter Aortic-Valve Replacement (TAVR). This figure underscores the region’s rising prominence within the TAVR market. Key drivers of market growth include an aging population, technological advancements, and increased healthcare expenditure. However, challenges such as regulatory hurdles and cost constraints persist, limiting market expansion in certain areas. Despite these challenges, opportunities abound, particularly in untapped markets and through strategic partnerships. Established multinational companies like Edwards Lifesciences Corporation and Medtronic plc dominate the competitive landscape, alongside emerging local competitors.
Key Countries Coverd in APAC Region
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Key Players Analysis
In the APAC Transcatheter Aortic-Valve Replacement (TAVR) market, several key players hold significant influence and contribute to market dynamics. Among these players are multinational corporations like Medtronic Plc., Edwards Lifesciences, Bracco SpA, and Transcatheter Technologies GmbH, alongside other emerging competitors. Medtronic Plc., a leader in medical technology, aims to address the region’s growing demand for TAVR procedures through innovative product offerings tailored to diverse patient needs.
Similarly, Edwards Lifesciences, a prominent player in structural heart disease solutions, focuses on expanding its presence across key APAC countries to enhance patient access to life-saving TAVR procedures. Bracco SpA, known for its expertise in medical imaging, supports APAC healthcare providers with essential contrast media and imaging technologies for TAVR procedures, emphasizing quality and safety.
Additionally, Transcatheter Technologies GmbH drives innovation in the APAC TAVR market through the development of advanced devices and procedural techniques, aiming to improve patient outcomes. Together with other market participants, these key players shape the future of TAVR in the APAC region through collaboration, innovation, and customer-centric approaches.
Market Key Players
- Medtronic Plc.
- Edwards Lifesciences
- Bracco SpA
- Transcatheter Technologies GmbH
- St. Jude Medical
- Meril Life Sciences
- Bracco SpA
- HLT Inc.
- SYMETIS SA
- Boston Scientific
- Other Key Players
Recent Developments
- In March 2024, Medtronic Plc.’s Evolut™ FX+ TAVR system gains FDA approval for treating symptomatic severe aortic stenosis, preserving valve benefits and enabling coronary access.
- In April 2023, Edwards Lifesciences introduces Cardi IQ Valve System in Japan, targeting mitral valve regurgitation treatment.
- In 2023, Meril Life Sciences partners with Japan Lifeline to market Myval Octacor, its advanced transcatheter heart valve, pending regulatory clearance in Japan.
- In December 2022, Boston Scientific’s ACURAX™ SC CoreValve™ Revascularization System obtains NMPA approval in China for severe aortic stenosis management.
Report Scope
Report Features Description Market Value (2023) USD 1.2 Bn Forecast Revenue (2033) USD 3.8 Bn CAGR (2024-2033) 12.2% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Balloon-expandable TAVR devices, Mechanically-expandable TAVR devices, Self-expanding TAVR devices), By Procedure Type (Transfemoral Implantation, Transapical Implantation, Transaortic Implantation), By End-Use (Hospitals, Specialty Clinics, Ambulatory Surgical Centers, Research/ Academic institutes) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Medtronic Plc., Edwards Lifesciences, Bracco SpA, Transcatheter Technologies GmbH, St. Jude Medical , Meril Life Sciences, Bracco SpA, HLT Inc., SYMETIS SA, Boston Scientific, Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the size of the APAC Transcatheter Aortic-Valve Replacement market in 2023?The APAC Transcatheter Aortic-Valve Replacement market size is USD 1.2 billion in 2023.
What is the projected CAGR at which the APAC Transcatheter Aortic-Valve Replacement market is expected to grow at?The APAC Transcatheter Aortic-Valve Replacement market is expected to grow at a CAGR of 12.2% (2024-2033).
List the segments encompassed in this report on the APAC Transcatheter Aortic-Valve Replacement market?Market.US has segmented the APAC Transcatheter Aortic-Valve Replacement market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Product Type the market has been segmented into Balloon-expandable TAVR devices, Mechanically-expandable TAVR devices, Self-expanding TAVR devices. By Procedure Type the market has been segmented into Transfemoral Implantation, Transapical Implantation, Transaortic Implantation. By End-Use the market has been segmented into Hospitals, Specialty Clinics, Ambulatory Surgical Centers, Research/ Academic institutes.
List the key industry players of the APAC Transcatheter Aortic-Valve Replacement market?Medtronic Plc., Edwards Lifesciences, Bracco SpA, Transcatheter Technologies GmbH, St. Jude Medical , Meril Life Sciences, Bracco SpA, HLT Inc., SYMETIS SA, Boston Scientific, Other Key Players
 APAC Transcatheter Aortic-Valve Replacement MarketPublished date: Apr 2024add_shopping_cartBuy Now get_appDownload Sample
APAC Transcatheter Aortic-Valve Replacement MarketPublished date: Apr 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Medtronic Plc.
- Edwards Lifesciences
- Bracco SpA
- Transcatheter Technologies GmbH
- St. Jude Medical
- Meril Life Sciences
- Bracco SpA
- HLT Inc.
- SYMETIS SA
- Boston Scientific
- Other Key Players










