Global Acute Bacterial Skin & Skin Structure Infections Treatment Market (ABSSI) By Infection Type (Hospital-Acquired ABSSI and Community-Acquired ABSSI), By Drug Type (Oral and Parental Antibiotics), By Route of Administration (Oral, Parental, and Topical), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2023-2032
- Published date: June 2024
- Report ID: 95316
- Number of Pages: 348
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
The Global Acute Bacterial Skin & Skin Structure Infections Treatment Market size is expected to be worth around USD 23.2 Bn by 2032 from USD 9.5 Bn in 2022, growing at a CAGR of 9.6% during the forecast period from 2022 to 2032.

Acute Bacterial Skin & Skin Structure Infections (ABSSSI) involve severe infections like cellulitis and abscesses, often caused by Staphylococcus aureus and Streptococcus pyogenes. Treatment typically includes oral or intravenous antibiotics, surgical interventions like incision and drainage, and supportive care such as pain management and wound care. Challenges include rising antibiotic resistance and ensuring patient compliance. Emerging therapies, such as new antibiotics and rapid diagnostics, aim to enhance treatment effectiveness and address these challenges, ensuring better outcomes for patients.
In the Acute Bacterial Skin & Skin Structure Infections (ABSSSI) treatment market, hospital pharmacies play a critical role, particularly in managing hospital-acquired infections that necessitate advanced antibiotic solutions. The market’s growth is propelled by the increasing incidence of infections resistant to conventional antibiotics, alongside a continuous demand for effective treatments in hospital settings. The U.S. FDA’s recent approval of new ABSSSI treatments highlights the region’s proactive stance in combating these healthcare challenges.
Stringent government regulations and initiatives in regions like the U.S. and Europe critically shape this market by ensuring the approval and monitoring of new therapeutic options aimed at tackling antimicrobial resistance. These rigorous regulatory frameworks facilitate the introduction of innovative treatments, which are vital for market growth. Additionally, the U.S. government supports these efforts through substantial funding from agencies like the Biomedical Advanced Research and Development Authority (BARDA). For example, Paratek Pharmaceuticals received approximately $77 million to support post-marketing studies for their FDA-approved drug.
Trade dynamics also significantly influence the ABSSSI treatment market, particularly in the U.S., which is a major importer and exporter of pharmaceuticals, including antibiotics. In 2023 alone, the U.S. imported antibiotics worth $200.95 million from China, underscoring a heavy reliance on global pharmaceutical trade chains. This trade is crucial not only for meeting domestic needs but also positions the U.S. as a significant player in the global pharmaceutical industry, affecting the availability and pricing of treatments.
Moreover, research and development focused on combating antibiotic resistance is a major market driver. Pharmaceutical giants like Merck & Co. and Pfizer are at the forefront, developing innovative antibiotics to combat resistant bacteria strains. These efforts address both hospital-acquired and community-acquired infections.
Recent strategic developments in the market include Melinta Therapeutics’ acquisition of Aurobindo Pharma’s antibiotics division, significantly boosting Melinta’s market presence. This acquisition, among other partnerships and product launches, highlights the dynamic nature of the market as companies strive to expand their portfolios and meet the evolving medical needs.
Key Takeaways
- Market Growth and Size: The global Acute Bacterial Skin & Skin Structure Infections treatment market is expected to grow from USD 9.5 billion in 2022 to around USD 23.2 billion by 2032, with a compound annual growth rate (CAGR) of 9.6% during this period.
- Infection Types: The market is segmented into hospital-acquired ABSSSI and community-acquired ABSSSI. Hospital-acquired ABSSSI is the fastest-growing segment, posing a significant risk to patient mortality. The market relies on effective antibiotic therapy to combat these infections.
- Drug Types: The market is categorized into oral and parental antibiotics. Newer antibiotics are gaining approval for treating Acute Bacterial Skin & Skin Structure Infections. Delafloxacin and Vancomycin are leading drugs in this market.
- Route of Administration: Parenteral administration is currently dominant due to its effectiveness against resistant bacteria. Oral and topical routes are also utilized, but parenteral products are preferred, ensuring a shorter treatment period.
- Distribution Channels: Hospital pharmacies hold the largest market share, followed by retail pharmacies and online pharmacies. Increased interaction with medical professionals drives demand for hospital pharmacies.
- Regional Analysis: North America continues to dominate the market due to extensive R&D, product pipelines, and regulatory approvals. The region has seen the launch of novel products and therapies for the treatment of these infections, contributing to market growth.
Infection Type Analysis
Hospital-acquired Acute Bacterial Skin & Skin Structure Infections (ABSSSI) typically manifest in patients during hospitalization, not being present upon their admission. These infections represent a significant challenge for healthcare facilities due to their contribution to elevated mortality rates globally. The increase in infections is notably influenced by Methicillin-resistant Staphylococcus aureus (MRSA), coupled with the growing resistance to this bacterium. This resistance trend is catalyzing the prevalence of hospital-acquired ABSSSI, underscoring the need for effective treatments.
The segment of hospital-acquired ABSSSI is projected to witness the fastest growth during the forecast period compared to other types. This rapid expansion is anticipated to significantly propel the overall market for treatments targeting Acute Bacterial Skin & Skin Structure Infections. Effective antibiotic therapy is crucial, targeting the most likely pathogens to eradicate the infections and support market growth.
Drug Type
Based on drug type, the global acute bacterial skin and skin structure infection market are segmented into oral and parental antibiotics. The Infectious Diseases Society of America (IDSA) has released guidelines categorizing ABSSSIs into nonpurulent and purulent types.
Nonpurulent infections, such as erysipelas and cellulitis, often require treatments like vancomycin and cefazolin, while purulent forms like abscesses might use agents such as doxycycline and trimethoprim-sulphamethoxazole. These guidelines prioritize different treatments based on severity and infection type, aligning with the Food and Drug Administration’s approval of antibiotics such as oritavancin and dalbavancin.
Delafloxacin holds the largest market share within the ABSSSI treatment sector due to its efficacy in treating various bacterial infections. Following closely is vancomycin, another highly utilized drug. The need for these treatments is driven by challenges such as soft tissue infections, resistance emergence, and complicated intra-abdominal infections.
Additionally, therapeutic drug monitoring is essential, especially in patients with renal impairment or those who are obese, to optimize the effectiveness and safety of these treatments. This strategic approach in drug administration underscores the evolving dynamics within the ABSSSI treatment landscape.
Route of Administration
Based on this segment, oral, topical, and parental are the routes of administration for acute bacterial skin and skin structure infection treatment products. This dominance is attributed to the introduction of novel products effective against methicillin-resistant Staphylococcus aureus (MRSA), the therapeutic benefits of parenteral treatment, and a shorter curative period compared to oral administration.
The market dominance of parenteral products is followed by the oral route, which holds a greater market share than topical treatments. Physicians must be vigilant in managing patients with known risk factors to optimize treatment outcomes.
A randomized controlled trial (RCT) found that switching from intravenous vancomycin to oral linezolid reduced the overall duration of therapy for patients with MRSA-associated skin and soft tissue infections (SSTIs), enhancing patient outcomes and reducing overall healthcare costs.
Additionally, a recent European retrospective study indicated that among patients with ABSSSIs, 20% experienced wound infections while around 80% had cellulitis. These findings suggest a significant drive towards growth in the ABSSSI treatment market, emphasizing the importance of effective management strategies and innovative treatment options.

Distribution Channel
The global Acute Bacterial Skin & Skin Structure Infections (ABSSSI) treatment market is segmented into retail pharmacy, hospital pharmacy, and online pharmacy based on the distribution channel. Among these, the hospital pharmacy segment registered the largest market share during the forecast period.
This can be attributed to the increased interaction between patients and medical prescribers, as well as other health professionals, which enhances the benefits and effectiveness of treatments. These interactions ensure that patients receive accurate prescriptions and necessary follow-ups, driving the demand for hospital pharmacies.
Following hospital pharmacies, retail pharmacies also generate substantial revenue, outperforming online pharmacies. The revenue from retail pharmacies is significant due to their widespread presence and the trust they have built among consumers over time. Retail pharmacies offer easy accessibility and immediate availability of medications, which are crucial for treating Acute Bacterial Skin & Skin Structure Infections.
In contrast, online pharmacies, while convenient, have not yet matched the revenue levels of hospital and retail pharmacies, partly due to delayed delivery times and the lack of direct interaction with healthcare professionals.
Key Market Segments
Based on Infection Type
- Hospital-acquired ABSSSIs
- Community-acquired ABSSSIs
Based on the Drug Type
- Oral Antibiotics
- Parental Antibiotics
Based on the Route of Administration
- Oral Route
- Parental Route
- Topical Route
Based on Distribution Channel Analysis
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Drivers
Increased R & D Activities By The Pharmaceutical Companies And Conduct Of Clinical Trials
The Global Acute Bacterial Skin and Skin Structure Infection (ABSSSI) Market is experiencing steady growth, with projections indicating significant expansion from 2023 to 2032. This growth is driven by increased research and development activities by pharmaceutical and biopharmaceutical companies, aiming to introduce novel products and treatments.
ABSSSI, which affects the dermis, epidermis, and subcutaneous tissue, manifests through symptoms such as wound infections, major cutaneous abscesses, burn infections, and extensive cellulitis, typically covering a minimum area of 75 cm². The involvement of pathogens like MRSA, MSSA, E. coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Streptococcus agalactiae underscores the importance of streptococcal antibiotics in treatment.
The U.S. Food and Drug Administration (FDA) actively supports clinical trials and programs for ABSSSI, contributing to advancements in treatment options. Additionally, the growing number of product approvals from regulatory authorities is expected to boost the market in North America and Europe. These regions are anticipated to see significant growth due to the consistent efforts in regulatory support and product innovation.
This optimistic market outlook highlights the essential role of continuous research and regulatory collaboration in addressing the challenges of Acute Bacterial Skin & Skin Structure Infections globally.
Restraints
Antimicrobial Resistance And Strict Regulatory Policies Slow Down The Market Growth Rate
Antimicrobial resistance presents a significant challenge for the long-term viability of antibiotic drugs in the market. This resistance leads to a reduction in the effectiveness of existing antibiotics, necessitating continuous innovation and development of new drugs by key market players.
The persistence of resistant bacteria diminishes the therapeutic value of established antibiotics, compelling pharmaceutical companies to invest in research and development to introduce new, more effective treatments. This ongoing battle against resistance is crucial to ensure that antibiotics remain a viable option for treating infections, highlighting the need for sustained efforts in drug innovation.
Additionally, regulatory policies also play a critical role in shaping the antibiotic market. The termination of patent licenses can significantly impact market growth, as it opens the market to generic versions of drugs, thereby reducing profits for the original developers. Such regulatory actions can discourage investment in antibiotic research due to the potential for diminished returns.
Consequently, the balance between encouraging new drug development and managing patent policies is essential for fostering a robust market that can address the challenges posed by antimicrobial resistance.
Opportunity
Rising Patient Pool
The development of the Acute Bacterial Skin & Skin Structure Infections (ABSSSI) market is anticipated to grow due to an increasing number of vagrant patients seeking treatment for these infections. Government bodies’ stringent administrative regulations, various strains of causative agents, and patent expirations are significant factors influencing this market.
For instance, in 2016, Allergan plc received US Food and Drug Administration (FDA) approval for its supplemental New Drug Application (sNDA) for Teflaro for treating ABSSSI in pediatric patients. Observational studies have shown that lesion size and infection duration are critical determinants of treatment failure probabilities.
The rising number of hospital-acquired ABSSSI presents a significant opportunity for key players to focus on introducing new drugs administered via the parenteral route, as these antibiotics are typically administered under medical supervision. When bacterial strains are resistant to treatment, surgical intervention is often required.
Additionally, the growing cases of antimicrobial resistance provide an opportunity for new entrants to develop innovative drugs. Key players can leverage these trends to expand their market presence by addressing these critical healthcare challenges.
Trends
Prevalence and Impact of Hospital-Acquired ABSSSI on Healthcare Systems
Hospital-acquired infections, also known as Healthcare-Associated Infections (HCAIs), are infections that patients contract during their stay in a hospital or healthcare facility. These infections were not present before the patients’ admission and can significantly prolong hospital stays.
According to a study titled “Update on the Epidemiology of Healthcare-Acquired Bacterial Infections: Focus on Complicated Skin and Skin Structure Infections,” published in the Journal of Antimicrobial Chemotherapy in November 2021, approximately 834,000 adult inpatients in National Health Service hospitals in England experience HCAIs annually. This accounts for 7.1 million bed days, which is 21 percent of the total annual bed days, indicating the substantial burden of these infections on the healthcare system.
The prevalence of hospital-acquired Acute Bacterial Skin & Skin Structure Infections (ABSSSI) is particularly notable. The same study estimates that 6.1% of these infections are hospital-acquired ABSSSI. Common types of these infections include Surgical Site Infections (SSIs) and Skin Soft Tissue Infections (SSTIs).
These infections can lead to extended hospital stays, increased healthcare costs, and significant impacts on patient outcomes. Therefore, it is anticipated that hospital-acquired ABSSSI will hold a sizable market share in the process component segment due to its high prevalence and the associated healthcare burden.
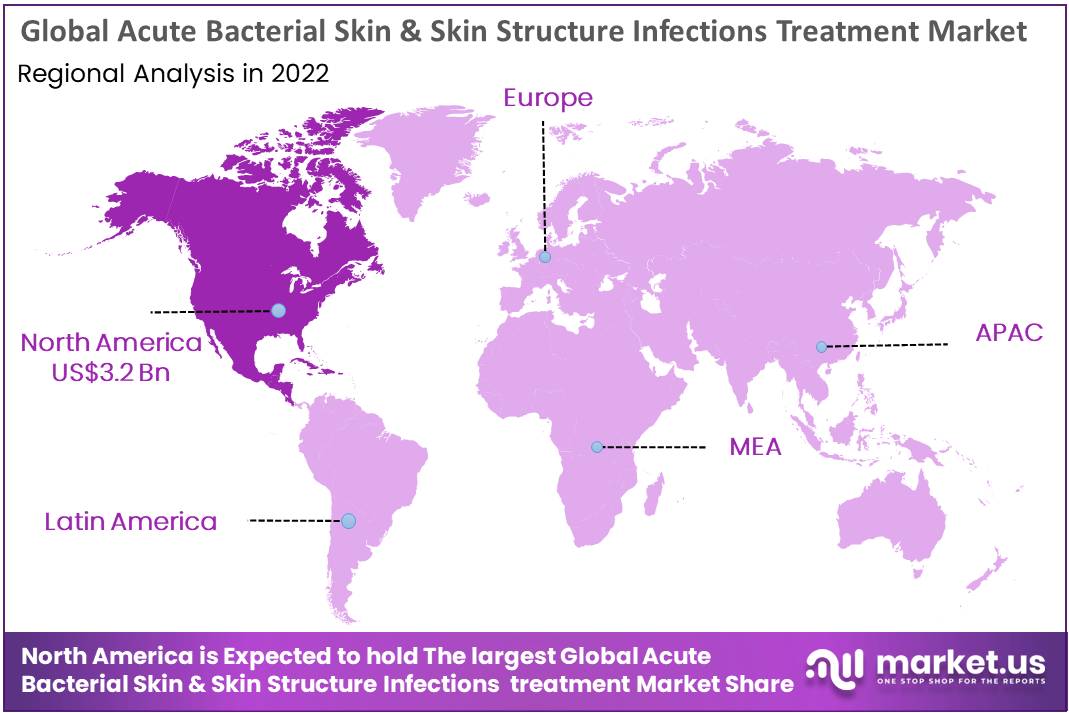
Regional Analysis
The global acute bacterial skin and skin structure infection treatment market is segmented into key regions, including North America, Latin America, Eastern Europe, Western Europe, Asia Pacific, and the Middle East & Africa.
North America is expected to maintain its dominance during the forecast period. This is attributed to increased development activity, extensive research, a robust product pipeline, novel product launches, and significant R&D spending. For instance, a study titled “Development of New Critical Pathway for Treatment of Acute Bacterial Skin & Skin Structure Infections,” posted by ClinicalTrials.gov in January 2020, highlights the clinical development of the drug Dalbavancin by Allergan Pharmaceuticals in the United States.
Furthermore, the number of drugs under clinical trials is expected to lead to more product launches over the prediction period. The growing product approval by regulatory authorities and an expanding product pipeline are also anticipated to boost market growth.
In 2022, Paladin Labs Inc., a subsidiary of Endo International plc, launched Xydalba (dalbavancin for injection) in Canada. This 30-minute IV therapy for Acute Bacterial Skin & Skin Structure Infections can be administered as a single or two-dose regimen. These factors are expected to drive the market in North America over the forecast period.
Key Regions
- North America
- The US
- Canada
- Mexico
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The Global Acute Bacterial Skin & Skin Structure Infections Treatment Market features prominent players such as Fresenius SE & Co. KGaA, Glenmark Pharmaceuticals Ltd., Melinta Therapeutics, Inc., and Allergan plc.
These companies are integral to the market, offering a range of treatment options and contributing to the industry’s growth through significant investments in research and development. Their efforts in expanding their product lines are crucial for addressing the diverse needs of patients with skin infections, enhancing their market positions and overall industry competitiveness.
Another set of key companies includes Teva Pharmaceutical Industries Ltd, Sandoz Inc. (a subsidiary of Novartis), Paratek Pharmaceuticals, Inc., Merck & Co. Inc., Accord Healthcare Ltd. (a subsidiary of Intas Pharmaceuticals Ltd.), Cadila Healthcare Ltd., and Aurobindo Pharma Ltd.
These market leaders are actively engaged in strategic collaborations, partnerships, and the development of new products to broaden their product portfolios. Their continuous innovation and strategic alliances play a vital role in advancing treatment options and meeting the growing demand for effective solutions in the Acute Bacterial Skin & Skin Structure Infections treatment market.
Market Key Players
- Paratek Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Melinta Therapeutics, Inc.
- AbbVie Inc.
- Sandoz International GmbH
- Glenmark Pharmaceuticals
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Limited
- Aceragen, Inc.
- Pfizer Inc.
- Allergen
- Cumberland Pharmaceutical Industries Ltd.
- Aurobindo Pharma Ltd.
- Accord Healthcare ltd. (a subsidiary of Intas Pharmaceuticals Ltd.)
- Cadila Healthcare Ltd.
- Other Key Players
Recent Developments
- In March 2024: Paratek Pharmaceuticals announced positive results for NUZYRA® (omadacycline) in treating inhalational anthrax, leading to additional BARDA Project BioShield contract procurement, supporting U.S. onshore manufacturing and bioterrorism threat mitigation.
- In September 2023: Merck & Co., Inc. acquired VelosBio for $2.75 billion to enhance its oncology pipeline, indirectly boosting research capabilities in infectious diseases, including ABSSSI treatments.
- In June 2023: Melinta Therapeutics launched KIMYRSA™ (oritavancin) for ABSSSI treatment. The single-dose regimen improves patient compliance and outcomes by providing rapid and sustained bacterial eradication.
- In April 2023: Pfizer partnered with BioNTech to develop mRNA-based vaccines targeting bacterial infections, including those causing ABSSSI, aiming to innovate treatments using advanced mRNA technology.
Report Scope
Report Features Description Market Value (2022) USD 9.5 Bn Forecast Revenue (2032) USD 23.3 Bn CAGR (2023-2032) 9.6% Base Year for Estimation 2023 Historic Period 2019-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments. Segments Covered By Infection – Hospital-Acquired ABSSI and Community-Acquired ABSSI; By Drug Type – Oral and Parental Antibiotics; By Route of Administration – Oral, Parental, and Topical; and By Distribution Channel – Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. Regional Analysis North America – The US, Canada, & Mexico; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA. Competitive Landscape Paratek Pharmaceuticals, Inc., Merck & Co., Inc., Melinta Therapeutics, Inc., AbbVie Inc., Sandoz International GmbH, Glenmark Pharmaceuticals, Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Limited, Aceragen, Inc., Pfizer Inc., Allergen, Cumberland Pharmaceutical Industries Ltd.Aurobindo Pharma Ltd., Accord Healthcare ltd. (a subsidiary of Intas Pharmaceuticals Ltd.), Cadila Healthcare Ltd., and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three license to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
Who are the prominent players in the global Acute Bacterial Skin & Skin Structure Infections Treatment Market?Paratek Pharmaceuticals, Inc. Merck & Co., Inc. Melinta Therapeutics, Inc. AbbVie Inc. Sandoz International GmbH Glenmark Pharmaceuticals Teva Pharmaceutical Industries Ltd. Sun Pharmaceutical Industries Limited Aceragen, Inc. Pfizer Inc. Allergen Cumberland Pharmaceutical Industries Ltd. Aurobindo Pharma Ltd. Accord Healthcare ltd. (a subsidiary of Intas Pharmaceuticals Ltd.) Cadila Healthcare Ltd. Other Key Players
How big was the global Acute Bacterial Skin & Skin Structure Infections Treatment Market in 2023?It was valued at USD 10 million in 2023
What is the growth rate of the global Acute Bacterial Skin & Skin Structure Infections Treatment Market?The global Acute Bacterial Skin & Skin Structure Infections Treatment Market is projected to grow at a CAGR of 9.60% between 2023 and 2032.
 Acute Bacterial Skin & Skin Structure Infections Treatment MarketPublished date: June 2024add_shopping_cartBuy Now get_appDownload Sample
Acute Bacterial Skin & Skin Structure Infections Treatment MarketPublished date: June 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Paratek Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Melinta Therapeutics, Inc.
- AbbVie Inc.
- Sandoz International GmbH
- Glenmark Pharmaceuticals
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Limited
- Aceragen, Inc.
- Pfizer Inc.
- Allergen
- Cumberland Pharmaceutical Industries Ltd.
- Aurobindo Pharma Ltd.
- Accord Healthcare ltd. (a subsidiary of Intas Pharmaceuticals Ltd.)
- Cadila Healthcare Ltd.
- Other Key Players










